* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Assessment of Left Ventricular Mass by Cardiovascular Magnetic
Cardiovascular disease wikipedia , lookup
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Electrocardiography wikipedia , lookup
Coronary artery disease wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
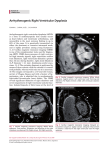
Assessment of Left Ventricular Mass by Cardiovascular Magnetic Resonance Saul G. Myerson, Nicholas G. Bellenger, Dudley J. Pennell Abstract—Left ventricular hypertrophy is associated with significant excess mortality and morbidity. The study and treatment of this condition, in particular the prognostic implications of changes in left ventricular mass, require an accurate, safe, and reproducible method of measurement. Cardiovascular magnetic resonance is a suitable tool for this purpose, and this review assesses the technique in comparison with others and examines the clinical and research implications of the improved reproducibility. (Hypertension. 2002;39:750-755.) Key Words: myocardium 䡲 hypertrophy 䡲 magnetic resonance imaging Downloaded from http://hyper.ahajournals.org/ by guest on May 3, 2017 T he importance of left ventricular hypertrophy (LVH) in medicine is not widely appreciated. The Framingham Study, among others, showed that increased left ventricular (LV) mass is associated with a significant excess of cardiovascular mortality and morbidity.1 This is independent of the presence of coronary artery disease2 or hypertension,1 with a tripling of the mortality rate in subjects with1–3 and without2 either of these. The risks of coronary, peripheral, or cerebrovascular disease are also raised, even among normotensive subjects with LVH.1,4,5 The accurate measurement of LV mass has in the past been difficult, partly because of the oblique angle at which the heart lies within the chest, its continuous movement, and the lack of a technique for imaging the whole left ventricle. Initial measurements with ECG data were surrogate markers for LV mass, with values affected by positioning of the leads, orientation of the heart, and obesity.6 – 8 Nevertheless, criteria were developed for identifying LVH with ECG9,10 that correlated to an extent with true LVH but were insensitive and nonspecific (specificity, 6% to 56%).11–13 Imaging techniques have now supplanted the ECG, and we review these with particular reference to cardiovascular magnetic resonance (CMR). geometric shape for both M-mode and 2D echo may lead to error, particularly as variations in ventricular geometry affect calculated LV mass.19 The landmark trials, such as Framingham,1 overcame the deficiencies in accuracy and reproducibility of echo with large numbers of subjects. M-mode is the commonest echocardiographic method for measuring LV mass, the images being easier to obtain and the calculations straightforward. Although validated against postmortem mainly normal hearts,20,21 it suffers most from the assumption of geometric shape, and this variability is reflected in the poor accuracy of the technique, with standard errors of the estimate (SEE) of 29 to 97 g (95% confidence interval [CI], 57 to 190 g).20 –23 Interstudy reproducibility is also poor, with SDs of the difference between successive measurements of 22 to 40 g (95% CI, 45 to 78 g).21,24 –27 The importance of operator skill is underlined by the large interobserver variability of a similar degree (SEE, 28 to 41 g; 95% CI, 55 to 80 g).21,24,25 2D echo has advantages over M-mode echo, as measurement is made of the ventricular length and minor axis in 2 planes. It still, however, assumes a prolate ellipsoid shape of the left ventricle and, to an extent, uniform wall thickness and is thus prone to similar inaccuracies as M-mode echo. The accuracy (SEE, 31 to 39 g)22,23 and reproducibility28 –30 are moderately improved over those of M-mode, although the increased difficulty in obtaining suitable quality images for evaluation may limit the ability to determine LV mass. 3D echo removes the assumption of shape and wall thickness. It has been shown to be more accurate than 2D or M-mode echo22,31 and is comparable to CMR,32,33 with reasonable reproducibility (95% CI, ⫾45 g),34 particularly with the newer transesophageal 3D echo (95% CI, ⫾12.8 g).35 The technique requires skill and time; however, and a number of subjects may not have suitable acoustic windows. Echocardiography Echocardiography (echo) was a distinct advance for LV mass measurement over the ECG, with direct visualization of the myocardium and real-time imaging, and many important studies examining the prognostic effects of LVH have used this method.1,2,14 However, obtaining good quality images is dependent on a skilled operator, patient position and anatomy, obesity, and angle of the transducer beam,15,16 and images of sufficient quality for LV mass measurement may not be obtained in up to one third of cases.16 –18 The assumed Received July 23, 2001; first decision August 4, 2001; revision accepted December 12, 2001. From the Cardiovascular Magnetic Resonance Unit, Royal Brompton Hospital, London, United Kingdom. Correspondence to Prof Dudley Pennell, Cardiovascular Magnetic Resonance Unit, Royal Brompton Hospital, Sydney St, par London SW3 6NP, United Kingdom. E-mail [email protected] © 2002 American Heart Association, Inc. Hypertension is available at http://www.hypertensionaha.org 750 Myerson et al LV Mass With Cardiovascular MR 751 There are currently relatively few units worldwide practicing the technique on a regular basis, and the transesophageal route may not be acceptable to some subjects. Electron-Beam Computed Tomography Downloaded from http://hyper.ahajournals.org/ by guest on May 3, 2017 The fast imaging time of electron beam computed tomography (EBCT), when coupled with blood-pool contrast agents, has facilitated 3D cardiac imaging precise enough to measure LV mass. The technique is similar to 3D echo and CMR in that multiple contiguous image planes are summed to measure myocardial volume, employing Simpson’s rule. When multiplied by the density of myocardial tissue (1.05 g/cm3), the LV mass is obtained. The ability to acquire many slices in a single breath-hold is also an advantage. There is good reported accuracy36,37 (SEE, 16 g in humans) and reproducibility,38 although human validation studies are limited. The disadvantages are the exposure to ionizing radiation and need for an intravenous contrast agent to delineate the cardiac blood pool. In addition, the image slices are not true short axes but an approximation, because of the limitations of available image planes, which decrease accuracy because of partial volume effects. Cardiovascular Magnetic Resonance 3D techniques such as EBCT and 3D echo are clearly better than 1D or 2D methods with assumptions about ventricular shape. CMR shares this advantage without the ionizing radiation or need for contrast agents with EBCT and without the problems of acoustic windows for echo. The free choice of imaging planes and good tissue visualization mean that virtually all images are of sufficient quality for LV mass determination. CMR Technique For the most accurate measurements, the image stack should be parallel to the true LV short axis, minimizing partial volume errors (Figure). The short axis is identified by first piloting the vertical long axis (VLA) plane from transaxial images, passing through the center of the mitral valve and apex of the LV. A horizontal long axis (HLA) plane is then obtained perpendicular to the VLA, again passing through the center of the mitral valve and apex. From the HLA, a stack of short-axis images is obtained, covering the length of the LV. ECG-gated cine CMR is acquired to measure LV mass at a single time point within the cardiac cycle (the standard is end-diastole). In addition, acquiring each image slice within a single breath-hold removes respiratory artifact. Usually 10 slices will cover the ventricle, and with a 10-second breathhold per slice, this can be achieved in ⬍10 minutes. With the newest scanners, cine stack can be obtained in a single breath-hold, reducing the time taken for a scan to ⬇8 seconds.39 The image stack can also provide volume information by summing the endocardial area on each slice to derive left (and right) ventricular volumes. The cine nature of the images allows end-diastolic and end-systolic volume to be measured and thus also stroke volume, as well as regional ventricular function. Diagrammatic representation of the LV with typical CMR short axis images obtained. Accuracy and Reproducibility The accuracy of CMR measurements of LV mass has been validated using postmortem hearts, imaged ex vivo for humans26,40 or in vivo for animal studies41– 46 (Table 1). These show good agreement between the CMR-obtained and true LV masses, with SD of the difference of ⬇8 g (95% CI, ⬇15 g) in human studies and 10 g (95% CI, ⬇19 g) in canine studies. The gold-standard validation of comparing in vivo images with subsequent postmortem weights has not been performed in humans, and this important comparison remains to be done. The reproducibility of LV mass measurements is of importance for assessing changes over time, both for individuals and research studies. This encompasses interstudy (ie, testretest reliability) and inter- and intraobserver variability in values. Again these are very good for CMR (Table 2),26,27,40,47–53 with interstudy variability having a mean weighted SD of the difference of 7.8 g (95% CI, 15.3 g).26,27,47,48 Mean weighted intra- and interobserver variabilities are 4.8 and 9.0 g, respectively.47,53 By comparison, the mean weighted interstudy SD of the difference for M-mode, 2D, and 3D echo is 27.7,21,24 –28 19.2,28,30 and 19.2 g,34,35 respectively. Clinical Implications The greater accuracy and reproducibility of 3D techniques, such as CMR, has important implications for clinical practice and research. The improved reproducibility means that in group studies, much smaller sample sizes can be used to detect the same change in LV mass. Alternatively, using the same sample size, smaller degrees of change can be identified. A comparison between CMR and echo of the sample 752 Hypertension March 2002 TABLE 1. Accuracy of CMR-Determined LV Mass in Human and Animal Studies No. SDD 95% CI Mean Difference Mean % Difference Notes 6 8.9 g ⫾17.5 g 0.7 g 4.0% Human Katz et al40 10 7.4 g ⫾14.5 g 10.2 g 5.3% Human Human studies (mean values) 16 8.0 g ⫾15.7 g McDonald et al41 10 1.8 g ⫾3.5 g 4.4 g 5.2% Shapiro et al42 10 6.7 g* ⫾13.1 g 8 8.7 g* ⫾17.1 g Caputo et al43 13 13.7 g* ⫾26.9 g Keller et al44 Study Bottini et al26 Canine Canine normal Canine post-MI 10.0% Canine normal ⫹ LVH Downloaded from http://hyper.ahajournals.org/ by guest on May 3, 2017 10 3.5 g ⫾6.9 g Maddahi et al45 8 4.9 g* ⫾9.6 g Canine; in vivo 9 3.4 g* ⫾6.7 g Canine; dead (in-situ) Florentine et al46 11 13.1 g* ⫾25.7 g Canine ⫹ feline Canine studies (mean values) 79 7.0 g ⫾13.7 g 6.8 g 13.3% Canine Values are compared to postmortem derived LV mass. Mean values are weighted for sample size. SDD indicates standard deviation of the difference between the 2 measurements; CI, confidence interval (1.96 ⫻ SDD); and MI, myocardial infarction. *SE of the estimate from regression equation. sizes needed to detect a statistically significant change in mean LV mass of 10 g are shown in Table 3. The numbers needed with CMR are ⬇8% of those with M-mode and 17% of those with 2D echo, with considerable savings in cost and study duration. CMR has already been used in clinical trials to identify very small differences in LV mass between groups: 9 to 11 g/m2 with 15 to 20 subjects per group54,55 and 7 g with groups of 30 to 40 subjects each.56 For individual patients, the 95% CI for serial studies using M-mode echo of ⫾45 to 78 g21,24 –27 means that serial LV TABLE 2. mass measurements cannot detect a change of less than this amount with any certainty. Given that most therapeutic interventions are likely to effect a change that is smaller than this, M-mode echo would not be an ideal method for serial changes. 2D echo has improved reproducibility, which results in better confidence intervals (⫾39 g),30 and these are ⫾13 to 45 g for 3D echo, depending on the route used (transesophageal versus transthoracic).34,35 CMR has 95% CIs of ⫾12 to 22 g,26,27,47,48 and this or another 3D technique should be used for individual changes in LV mass. Reproducibility of CMR-Derived LV Mass Measurements from Human Studies Study No. Interstudy Intraobserver Interobserver Notes 15 6.4 g (2.8%) 3.0 g (1.6%) 5.1 g (2.4%) Normal subjects 15 6.4 g (3.0%) 5.9 g (2.7%) 7.7 g (3.1%) 4 8.2 g Normal subjects Germain et al27 20 11.2 g (6.7%) Normal subjects Grothues et al48 20 4.2 g (2.3%) Normal subjects 20 9.6 g (3.8%) Heart failure 20 8.4 g (2.8%) LVH Bellenger et al 47 Bottini et al26 Semelka et al Heart failure 49 11 5.2% 4.4% Normal subjects Semelka et al50 11 4.7–6.1% 3.4% DCM 8 3.5–4.8% 5.5% LVH 12 4.4% 4.2% Normal subjects Bogaert et al51 Matheijssen et al52 4.1% 8 3.6% 3.6% MI Yamaoka et al53 10 5.8 g 17.8 g Normal, LVH, and DCM Katz et al40 10 Mean weighted values 7.8 g (n⫽114) 6.1% 7.2% 4.8 g (n⫽40) 9.0 g (n⫽40) Values are standard deviations of the difference between successive scans (g) or % variability. Mean values are for studies with absolute values, weighted by sample size. DCM indicates dilated cardiomyopathy; MI, myocardial infarction. Myerson et al TABLE 3. Comparison of Sample Sizes Needed to Detect a Statistically Significant Change in Mean LV Mass of 10 g Power (1- Error) CMR 2D Echo M-Mode Echo 99% 23 136 283 95% 16 96 199 90% 13 78 162 80% 10 58 121 These assume a 2-tailed unpaired t test with 0.05 level of significance (␣-error) and refer to the sample size in each group (2 parallel treatment groups would need twice this number in total). We used the mean weighted values of interstudy standard deviation of the difference for M-mode echo (27.7 g),21,24 –28 2D echo (19.2 g),28,30 and CMR (7.8 g).26,27,47,48 Limitations of CMR Downloaded from http://hyper.ahajournals.org/ by guest on May 3, 2017 Patient factors can sometimes limit the usefulness of the technique. Because of the enclosed nature of the CMR scanner, some people find this too claustrophobic. In practice, the incidence of this is ⬇3% to 5%, though light intravenous anxiolysis with diazepam to can reduce this to 1%.57 Advances in equipment technology, with shorter and more open magnets together with reduced time in the scanner from faster imaging, will also improve conditions for these patients. The same restrictions as for any MR scanner apply for patients with cranial aneurysm clips, ocular metallic shards, and pacemakers. The need for breath-holding to remove respiratory motion artifact can present problems for some patients with severe cardiac or respiratory disease; for these patients, “navigator” sequences can be used in which free breathing is allowed and the diaphragm is continuously monitored, with imaging adjusted for the diaphragm position.58 This has the disadvantage of slightly increased imaging time, but image quality is well maintained. Currently, the availability of CMR scanners capable of cardiac work and the skilled personnel needed to obtain and interpret the images limits the widespread clinical use of this technique, although many pharmacological studies with CMR have already been performed. This is likely to change in the near future, with nearly all new MR scanners having the required hardware and the cardiac software. Although the initial cost of the scanner is high, for research purposes the savings from the reduced number of subjects may offset this substantially. A single study takes ⬇15 minutes for postprocessing with standard techniques. Although this is longer than for echo, the new generation of machines coupled with automated image processing will greatly reduce these times and will allow a fast-throughput service. Conclusions LVH is a potent risk factor for cardiovascular disease and is associated with significant increases in morbidity and mortality, but traditionally little has been done to specifically address this issue. This is because until recently, no reliable and reproducible technique existed to quantify LV mass. We now know that the measurement of LV mass is best accomplished with 3D techniques, and CMR has become the gold standard. 3D echo and EBCT achieve an accuracy and reproducibility close to those of CMR and are acceptable LV Mass With Cardiovascular MR 753 alternatives, but problems preclude their regular use. Now that good data exist in particular for the effectiveness of ACE inhibitors in reducing LV mass,59 – 61 and the prognostic benefits of LV mass reduction are becoming defined,62– 64 coupled with a reproducible and accurate measurement technique in CMR, it is likely that more clinical and research attention will be paid to this condition. The fidelity of the CMR technique may also lead to new understanding of the precise mechanisms behind LVH,65 and why its effects on mortality are so profound, even in the absence of coronary artery disease and hypertension, although recent work implicates plaque disruption as an important issue.66 Acknowledgments The British Heart Foundation provided funding for Dr Myerson (FS 97030). This work was also supported by the Coronary Artery Disease Research Association (CORDA) and the Wellcome Trust. References 1. Levy D, Garrison R, Savage D, Kannel W, Castelli W. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. 2. Ghali J, Liao Y, Simmons B, Castaner A, Cao G, Cooper R. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med. 1992;117:831– 836. 3. Kaplinsky E. Significance of left ventricular hypertrophy in cardiovascular morbidity and mortality. Cardiovasc Drugs Ther. 1994;8:549 –556. 4. Roman M, Pickering T, Schwartz J, Pini R, Devereux R. Association of carotid atherosclerosis and left ventricular hypertrophy. J Am Coll Cardiol. 1995;25:83–90. 5. Kannel W, Cobb J. Left ventricular hypertrophy and mortality: results from the Framingham Study. Cardiology. 1992;81:291–298. 6. Riekkinen H, Rautaharju P. Body position, electrode level, and respiration effects on the Frank lead electrocardiogram. Circulation. 1976;53: 40 – 45. 7. Hoekema R, Uijen G, van Erning L, van Oosterom A. Interindividual variability of multilead electrocardiographic recordings: influence of heart position. J Electrocardiol. 1999;32:137–148. 8. Sugita S, Takada K, Hayano J. Influence of body composition on electrocardiographic identification of left ventricular hypertrophy in adolescents. Cardiology. 1999;91:127–133. 9. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. 10. Romhilt D, Estes EJ. A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J. 1968;75:752–758. 11. Romhilt D, Bove K, Norris R, Conyers E, Conradi S, Rowlands D, Scott R. A critical appraisal of the electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. Circulation. 1969;40:185–195. 12. Reichek N, Devereux R. Left ventricular hypertrophy: relationship of anatomic, echocardiographic and electrocardiographic findings. Circulation. 1981;63:1391–1398. 13. Woythaler J, Singer S, Kwan O, Meltzer R, Reubner B, Bommer W, DeMaria A. Accuracy of echocardiography versus electrocardiography in detecting left ventricular hypertrophy: comparison with postmortem mass measurements. J Am Coll Cardiol. 1983;2:305–311. 14. Massie BM, Tubau JF, Szlachcic J, O’Kelly BF. Hypertensive heart disease: the critical role of left ventricular hypertrophy. J Cardiovasc Pharm. 1989;13:S18 –S24. 15. Wallerson D, Devereux R. Reproducibility of echocardiographic left ventricular measurements. Hypertension. 1987;9(suppl II):II-6 –II-18. 16. Wong M, Shah P, Taylor R. Reproducibility of left ventricular internal dimensions with M mode echocardiography: effects of heart size, body position and transducer angulation. Am J Cardiol. 1981;47:1068 –1074. 17. Valdez R, Motta J, London E, Martin R, Haskell W, Farquhar J, Popp R, Horlick L. Evaluation of the echocardiogram as an epidemiologic tool in an asymptomatic population. Circulation. 1979;60:921–929. 18. Gardin J, Arnold A, Gottdiener J, Wong N, Fried L, Klopfenstein H, O’Leary D, Tracy R, Kronmal R. Left ventricular mass in the elderly: The Cardiovascular Health Study. Hypertension. 1997;29:1095–1103. 754 Hypertension March 2002 Downloaded from http://hyper.ahajournals.org/ by guest on May 3, 2017 19. Devereux R, Casale P, Wallerson D, Kligfield P, Hammond I, Liebson P, Campo E, Alonso D, Laragh J. Cost-effectiveness of echocardiography and electrocardiography for detection of left ventricular hypertrophy in patients with systemic hypertension. Hypertension. 1987;9(suppl II):II69 –II-76. 20. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man: anatomic validation of the method. Circulation. 1977;55:613– 619. 21. Devereux R, Alonso D, Lutas E, Gottlieb G, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450 – 458. 22. Gopal A, Schnellbaecher M, Shen Z, Akinboboye O, Sapin P, King D. Freehand three-dimensional echocardiography for measurement of left ventricular mass: in vivo anatomic validation using explanted human hearts. J Am Coll Cardiol. 1997;30:802– 810. 23. Reichek N, Helak J, Plappert T, Sutton M, Weber K. Anatomic validation of left ventricular mass estimates from clinical two-dimensional echocardiography: initial results. Circulation. 1983;67:348 –352. 24. Gottdiener J, Livengood S, Meyer P, Chase G. Should echocardiography be performed to assess effects of antihypertensive therapy?: test-retest reliability of echocardiography for measurement of left ventricular mass and function. J Am Coll Cardiol. 1995;25:424 – 430. 25. Gosse P, Roudaut R, Dallocchio M. Is echocardiography an adequate method to evaluate left ventricular hypertrophy regression? Eur Heart J. 1990;11:107–112. 26. Bottini P, Carr A, Prisant L, Flickinger F, Allison J, Gottdiener J. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8: 221–228. 27. Germain P, Roul G, Kastler B, Mossard J, Bareiss P, Sacrez A. Inter-study variability in left ventricular mass measurement: comparison between M-mode echocardiography and MRI. Eur Heart J 1992;13: 1011–1019. 28. Collins H, Kronenberg M, Byrd B. Reproducibility of left ventricular mass measurements by two-dimensional and M-mode echocardiography. J Am Coll Cardiol. 1989;14:672– 676. 29. Himelman R, Cassidy M, Landzberg J, Schiller N. Reproducibility of quantitative two-dimensional echocardiography. Am Heart J. 1988;115: 425– 431. 30. Palmieri V, Dahlof B, DeQuattro V, Sharpe N, Bella J, de Simone G, Paranicas M, Fishman D, Devereux R. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE Study. J Am Coll Cardiol. 1999;34:1625–1632. 31. Gopal A, Keller A, Shen Z, Sapin P, Schroeder K, King DJ, King D. Three-dimensional echocardiography: in vitro and in vivo validation of left ventricular mass and comparison with conventional echocardiographic methods. J Am Coll Cardiol. 1994;24:504 –513. 32. Nosir Y, Lequin M, Kasprzak J, van Domburg R, Vletter W, Yao J, Stoker J, Ten Cate F, Roelandt J. Measurements and day-to-day variabilities of left ventricular volumes and ejection fraction by threedimensional echocardiography and comparison with magnetic resonance imaging. Am J Cardiol. 1998;82:209 –214. 33. Altmann K, Shen Z, Boxt L, King D, Gersony W, Allan L, Apfel H. Comparison of three-dimensional echocardiographic assessment of volume, mass and function in children with functionally single left ventricles with two-dimensional echocardiography and magnetic resonance imaging. Am J Cardiol. 1997;80:1060 –1066. 34. Gopal A, Schnellbaecher M, Shen Z, Sciacca R, Keller A, Sapin P, King D. Serial assessment of left ventricular mass regression by 3D echocardiography requires three-fold fewer subjects compared to conventional 1D and 2D echocardiography. J Am Coll Cardiol. 1996;81:150A. Abstract. 35. Kuhl H, Bucker A, Franke A, Maul S, Nolte-Ernsting C, Reineke T, Hoffmann R, Gunther R, Hanrath P. Transesophageal 3-dimensional echocardiography: in vivo determination of left ventricular mass in comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 2000; 13:205–215. 36. Feiring A, Rumberger J, Reiter S, Skorton D, Collins S, Lipton M, Higgins C, Ell S, Marcus M. Determination of left ventricular mass in dogs with rapid-acquisition cardiac computed tomographic scanning. Circulation. 1985;72:1355–1364. 37. Mousseaux E, Beygui F, Fornes P, Chatellier G, Hagege A, Desnos M, Lecomte D, Gaux J. Determination of left ventricular mass with electron beam computed tomography in deformed, hypertrophic human hearts. Eur Heart J. 1994;15:832– 841. 38. Schmermund A, Rensing B, SHeedy P, Rumberger J. Reproducibility of right and left ventricular volume measurements by electron-beam CT in patients with congestive heart failure. Int J Card Imaging. 1998;14: 201–209. 39. Motooka M, Matsuda T, Kida M, Inoue H, Hayashi K, Takahashi T, Sasayama S. Single breath-hold left ventricular volume measurement by 0.3-sec turbo fast low-angle shot MR imaging. Am J Roentgenol. 1999; 172:1645–1649. 40. Katz J, Milliken M, Stray-Gundersen J, Buja L, Parkey R, Mitchell J, Peshock R. Estimation of human myocardial mass with MR imaging. Radiology. 1988;169:495– 498. 41. McDonald K, Parrish T, Wennberg P, Stillman A, Francis G, Cohn J, Hunter D, Rapid, accurate and simultaneous noninvasive assessment of right and left ventricular mass with nuclear magnetic resonance imaging using the snapshot gradient method. J Am Coll Cardiol. 1992;19: 1601–1607. 42. Shapiro E, Rogers W, Beyar R, Soulen R, Zerhouni E, Lima J, Weiss J. Determination of left ventricular mass by magnetic resonance imaging in hearts deformed by acute infarction. Circulation. 1989;79:706 –711. 43. Caputo G, Tscholakoff D, Sechtem U, Higgins C. Measurement of canine left ventricular mass by using MR imaging. Am J Roentgenol. 1987;148: 33–38. 44. Keller A, Peshock R, Malloy C, Buja L, Nunnally R, Parkey R, Willerson J. In vivo measurement of myocardial mass using nuclear magnetic resonance imaging. J Am Coll Cardiol. 1986;8:113–117. 45. Maddahi J, Crues J, Berman D, Mericle J, Becerra A, Garcia E, Henderson R, Bradley W. Noninvasive quantification of left ventricular myocardial mass by gated proton nuclear magnetic resonance imaging. J Am Coll Cardiol. 1987;10:682– 692. 46. Florentine M, Grosskreutz C, Chang W, Hartnett J, Dunn V, Ehrhardt J, Fleagle S, Collins S, Marcus M, Skorton D. Measurement of left ventricular mass in vivo using gated nuclear magnetic resonance imaging. J Am Coll Cardiol. 1986;8:107–112. 47. Bellenger NG, Davies LC, Francis JM, Coats ACS, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–278. 48. Grothues F, Smith GS, Bellenger NG, Collins P, Klein H, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance and 2D-echocardiography in normals, patients with congestive heart failure and in patients with left ventricular hypertrophy. Am J Cardiol. In press. 49. Semelka R, Tomei E, Wagner S, Mayo J, Kondo C, Suzuki J, Caputo G, Higgins C. Normal left ventricular dimensions and function: interstudy reproducibility of measurements with cine MR imaging. Radiology. 1990; 174:763–768. 50. Semelka R, Tomei E, Wagner S, Mayo J, Caputo G, O’Sullivan M, Parmley W, Chatterjee K, Wolfe C, Higgins C. Interstudy reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle. Am Heart J. 1990;119:1367–1373. 51. Bogaert J, Bosmans H, Rademakers F, Bellon E, Herregods M, Verschakelen J, Van de Werf F, Marchal G. Left ventricular quantification with breath-hold MR imaging: comparison with echocardiography. MAGMA. 1995;3:5–12. 52. Matheijssen N, Baur L, Reiber J, van der Velde E, van Dijkman P, van der Geest R, de Roos A, van der Wall E. Assessment of left ventricular volume and mass by cine magnetic resonance imaging in patients with anterior myocardial infarction intra-observer and inter-observer variability on contour detection. Int J Card Imaging. 1996;12:11–19. 53. Yamaoka O, Yabe T, Okada M, Endoh S, Nakamura Y, Mitsunami K, Kinoshita M, Mori M, Murata K, Morita R. Evaluation of left ventricular mass: comparison of ultrafast computed tomography, magnetic resonance imaging, and contrast left ventriculography. Am Heart J. 1993;126: 1372–1379. 54. Gaudio C, Tanzilli G, Ferri F, Villatico Campbell S, Bertocchi F, Motolese M, Campa P. Benazepril causes in hypertension a greater reduction in left ventricular mass than does nitrendipine: a randomized study using magnetic resonance imaging. J Cardiovasc Pharmacol. 1998; 32:760 –768. 55. Johnson D, Foster R, Barilla F, Blackwell G, Roney M, Stanley AJ, Kirk K, Orr R, van der Geest R, Reiber J, Dell’Italia L. Angiotensin-converting enzyme inhibitor therapy affects left ventricular mass in patients with ejection fraction ⬎40% after acute myocardial infarction. J Am Coll Cardiol. 1997;29:49 –54. Myerson et al 56. Myerson SG, Montgomery H, Whittingham M, Jubb M, World M, Humphries S, Pennell DJ. Left ventricular hypertrophy with exercise and the angiotensin converting enzyme gene I/D polymorphism: a randomized controlled trial with losartan. Circulation. 2001;103:226 –230. 57. Francis JM, Pennell DJ. The treatment of claustrophobia during cardiovascular magnetic resonance: use and effectiveness of mild sedation. J Cardiovasc Magn Reson. 2000;2:139 –141. 58. Bellenger N, Gatehouse P, Rajappan K, Keegan J, Firmin D, Pennell D. Left ventricular quantification in heart failure by cardiovascular MR using prospective respiratory navigator gating: comparison with breath-hold acquisition. J Magn Reson Imaging. 2000;11:411– 417. 59. Roman M, Alderman M, Pickering T, Pini R, Keating J, Sealey J, Devereux R. Differential effects of angiotensin converting enzyme inhibition and diuretic therapy on reductions in ambulatory blood pressure, left ventricular mass, and vascular hypertrophy. Am J Hypertens. 1998; 11:387–396. 60. Dahlof B, Pennert K, Hansson L. Reversal of left ventricular hypertrophy in hypertensive patients: a metaanalysis of 109 treatment studies. Am J Hypertens. 1992;5:95–110. 61. Lievre M, Gueret P, Gayet C, Roudaut R, Haugh MC, Delair S, Boissel JP. Ramipril-induced regression of left ventricular hypertrophy in treated hypertensive individuals. Hypertension. 1995;25:92–97. LV Mass With Cardiovascular MR 755 62. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Reboldi G, Porcellati C. Prognostic significance of changes in left ventricular mass in essential hypertension. Circulation. 1998;97: 48 –54. 63. Levy D, Salomon M, D’Agostino R, Belanger A, Kannel W. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994; 90:1786 –1793. 64. Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S for the Heart Outcomes Prevention Evaluation (HOPE) Investigators: reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor Ramipril. Circulation. 2001;104:1615–1621. 65. Brull D, Dhamrait S, Woods D, Myerson S, Pennell DJ, World M, Humphries S, Regitz-Zagrosek V, Montgomery H. The bradykinin B2 receptor and the human left ventricular growth response. Lancet. 2001; 358:1155–1156. 66. Heidland UE, Strauer BE. Left ventricular muscle mass and elevated heart rate are associated with coronary plaque disruption. Circulation. 2001;104:1477–1482. Downloaded from http://hyper.ahajournals.org/ by guest on May 3, 2017 Assessment of Left Ventricular Mass by Cardiovascular Magnetic Resonance Saul G. Myerson, Nicholas G. Bellenger and Dudley J. Pennell Downloaded from http://hyper.ahajournals.org/ by guest on May 3, 2017 Hypertension. 2002;39:750-755 doi: 10.1161/hy0302.104674 Hypertension is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2002 American Heart Association, Inc. All rights reserved. Print ISSN: 0194-911X. Online ISSN: 1524-4563 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://hyper.ahajournals.org/content/39/3/750 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Hypertension can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Hypertension is online at: http://hyper.ahajournals.org//subscriptions/