* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Richardson Final miR Commentary Diabetes 2016
Survey
Document related concepts
Hospital-acquired infection wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Neonatal infection wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Molecular mimicry wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Infection control wikipedia , lookup
Innate immune system wikipedia , lookup
Hepatitis B wikipedia , lookup
Transcript
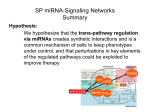
miR, miR in the cell, does the virus control them all? Sarah J Richardson1, Marc S Horwitz2 1 University of Exeter Medical School, Devon, UK 2 University of British Columbia, Vancouver, Canada Correspondence to: Marc Horwitz Associate Professor, Sauder Chair of Pediatric Virology Co-leader, Infection, Inflammation & Immunity Research Group Department of Microbiology and Immunology University of British Columbia 3551-2530 Health Sciences Mall, Vancouver, BC V6T 1Z3, Canada Tel: 604-822-6298 FAX: 604-822-6041 E-mail: [email protected] Dr Sarah Richardson Lecturer in Biomedical Sciences (Education & Research) Institute of Biomedical and Clinical Sciences University of Exeter Medical School RILD Building (Level 4), Barrack Road, Exeter, EX2 5DW, UK Tel: +44 (0)1392 408225 E-mail: [email protected] Word Count : 1000 Figures: 1 As if the pathway of regulation leading islet cells toward dysfunction and diabetes was not difficult enough to grasp, here come viruses to further complicate matters. In the past few years, we have come to recognize that a class of small noncoding RNAs termed microRNAs (miRNAs) has a powerful ability to regulate most, if not all, of the key processes in cell biology (1,2). In fact, there is evidence that these key molecules can be transported from cell to cell, thereby influencing gene expression not only within the host cell but also in neighboring cells and in distant cell subsets when the miRNAs travel in vesicles via the blood and lymph (3–5). The evidence that miRNAs play pivotal roles in b-cell biology, including regulating their differentiation, glucose responsiveness, insulin production, apoptosis, and inflammation, is rapidly accumulating and was recently discussed by Filios and Shalev (4) in an issue of Diabetes. As one might expect given their range of functions within the b-cells, these same molecules are hypothesized to have key roles in the pathogenesis of type 1 diabetes (4,6–13). Yet, it is still not clear whether or not they are central to the induction of disease or if they are merely a biomarker of active b-cell dysfunction. Type 1 diabetes is often described as a chronic autoimmune disease characterized by the destruction of insulin producing islet b-cells, and it is a widely held belief that a complex blend of genetic and environmental factors is required to drive disease. In terms of clinically relevant environmental factors, the culprits that are most often mentioned and have the strongest evidentiary support are coxsackieviruses (CVBs). Evidence for CVB infection has often been described in recent-onset patients, and CVBs have a clear propensity for infecting human islet b-cells and can induce diabetes in mouse models (14–16). Whereas many viruses encode their own miRNAs to control both viral and cellular processes, other viruses simply usurp cellular miRNAs to their own advantage (3,5,17). Small RNA viruses such as CVBs seem to lack miRNAs, as none have been identified in their genomes to date. Previously, several groups have examined the impact of cytokines on miRNAs in mouse and human b-cells (7,13,18), but the study by Kim et al. (19) in this issue of Diabetes is the first study to examine the impact of an enterovirus infection on miRNA expression in human pancreatic islets. Taking into consideration the important role of miRNAs in normal b-cell function, Kim et al. asked whether a virus infection could be in part responsible for the changes in miRNA expression within the infected b-cell that can subsequently contribute to a type 1 diabetes phenotype. These changes could promote b-cell dysfunction and/or apoptosis, could alter the sensing and subsequent antiviral host cell immune response, and could impact the ability of infiltrating and peripheral immune cells to respond to an infection, potentially promoting increased inflammation, surveillance, or antigen presentation. Kim et al. (19) assessed the cellular expression of miRNAs within human islets infected with CVB5. In total, the expression of 754 miRNAs was assessed in the infected islets using a highthroughput quantitative PCR array, with the expression of 33 miRNAs found to be significantly altered when compared with the control islets. Strikingly, these 33 miRNAs were predicted to target 57 of the 72 known type 1 diabetes risk genes. The majority of miRNAs were downregulated postinfection but 6 were upregulated (Fig. 1). Key immune modulatory miRNAs miR-155-5p (increased fourfold) and miR-181a-3p (decreased 4.4-fold) were found to be dysregulated by 24 h after infection, and intriguingly, miR-34a-5p demonstrated the greatest increase in expression (more than eightfold) of any miRNA by 4 days post-infection. miR-34a-5p is pluripotent and has been shown to target genes resulting in diminishing insulin secretion (VAMP2) and increasing apoptosis (BclII) (18). Additionally, miR-155-5p has also been shown by others to affect several other diabetes risk genes such as GLIS3 (b-cell survival) and SOCS1 (negative regulator of the type I interferon [antivirus] pathway) (8,9). Other dysregulated miRNAs were predicted to target a number of pathogen recognition receptors, such as IFIHI, TLR7, and TLR8; cytokines, including IL2, IL10, IL17D, and IL21; and regulators of cytokine apoptosis, such as BACH2. Many of these miRNA–target gene interactions have been validated in both adult pancreatic b-cells as well as b-cells from prediabetic mice (NOD and db/db) (18), but others require further verification. Clearly though, CVB5 infection alters cellular miRNA expression to focus the cell’s material and energy to promote virus replication while at the same time attempting to downregulate pathways that would inhibit virus propagation, including those affecting antivirus responses. The production of proteins that are not required for the virus life cycle, such as insulin and apoptosis pathways, appear to be downregulated. An example of the latter is miR-21, which is increased more than sevenfold in the infected cells and targets the tumor suppressor PDCD4, which has been shown to block pancreatic cell death in mouse models (12). Several miRNAs, including some identified by Kim et al. (19) (miR-21, miR-181, and miR-29a), were identi- fied as dysregulated in the serum of children with type 1 diabetes (10,11). Additionally, multiple reports have identified exosomes containing miRNAs that are exchanged between immune cells and result in changes in cellular expression, cytokine expression, and antigen presentation. An entire cluster of miRNA loci has been shown to target the expression of type 1 diabetes autoantigens IA-2, IA-2b, and GAD65 (6). It seems that virus infection reflects miRNA expression back onto the cell in an effort to prioritize replication of virus and on toward surrounding cells in an effort to minimize detection. In a susceptible individual, can this induce islet cell dysfunction and diabetes? This is the premise of the study by Kim et al. (19), yet a number of questions are raised. Prior work has demonstrated that different strains and substrains of CVB are associated with diabetes induction and protection (20). As such, the most strongly associated diabetogenic strains are CVB1 and CVB4, and their miRNA expression pattern may reveal further specific diabetes-associated miRNAs and targets. Likewise, the virus-mediated mechanism for the induction of diabetes remains elusive, yet two viable hypotheses endure and suggest that the disease could either be induced following an acute infection that is subsequently resolved or via a more chronic, persistent infection of b-cells. The expression of miRNA during acute and latent herpesvirus infection has been well described to change over the life cycle of the virus, and it would be expected that similar results would occur with CVB (5). Kim et al. describe the early events of acute infection with exhibited changes in miRNA expression within the first 7 days. Later time points during persistent infection would likely reveal a novel pattern of miRNA expression. Furthermore, exosome analysis of the intercommunication between cells could uncover communication within the islet to neighboring endocrine cells as well as with the immune cells responding to infection. Clearly, longitudinal studies of in vivo tissue from mice and humans will add to the confirmation of potential viral etiology. In summary, CVB infection of human islets dramatically alters the profile of miRNA expression. Many of the affected miRNAs target the type 1 diabetes risk genes, suggesting that viral infection has another means by which it could contribute to the development of a type 1 diabetes phenotype. Further array analysis for cellular and vesicular miRNAs will likely expose a number of potential miRNAs as therapies themselves or as therapeutic targets. Funding. This work was partially funded by a JDRF Career Development Award (5-CDA-2014-221-AN) to S.J.R. Duality of Interest. No potential conflicts of interest relevant to this article were reported. References 1. Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 2010;463:621–626 2. Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 2008;105:13421–13426 3. Cullen BR. Five questions about viruses and microRNAs. PLoS Pathog 2010; 6:e1000787 4. Filios SR, Shalev A. b-Cell microRNAs: small but powerful. Diabetes 2015; 64:3631–3644 5. Kincaid RP, Sullivan CS. Virus-encoded microRNAs: an overview and a look to the future. PLoS Pathog 2012;8:e1003018 6. Abuhatzira L, Xu H, Tahhan G, Boulougoura A, Schäffer AA, Notkins AL. Multiple microRNAs within the 14q32 cluster target the mRNAs of major type 1 diabetes autoantigens IA-2, IA-2b, and GAD65. FASEB J 2015;29:4374–4383 7. Fernandez-Valverde SL, Taft RJ, Mattick JS. microRNAs in b-cell biology, insulin resistance, diabetes and its complications. Diabetes 2011;60:1825– 1831 8. Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K, Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J Immunol 2009;182:2578– 2582 9. Lu LF, Thai TH, Calado DP, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 2009;30:80–91 10. Nielsen LB, Wang C, Sørensen K, et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res 2012;2012:896362 11. Osipova J, Fischer DC, Dangwal S, et al. Diabetes-associated microRNAs in pediatric patients with type 1 diabetes mellitus: a cross-sectional cohort study. J Clin Endocrinol Metab 2014;99:E1661– E1665 12. Ruan Q, Wang T, Kameswaran V, et al. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic beta cell death. Proc Natl Acad Sci U S A 2011;108:12030–12035 13. Ventriglia G, Nigi L, Sebastiani G, Dotta F. microRNAs: novel players in the dialogue between pancreatic islets and immune system in autoimmune diabetes. BioMed Res Int 2015;2015:749734 14. Drescher KM, von Herrath M, Tracy S. Enteroviruses, hygiene and type 1 diabetes: toward a preventive vaccine. Rev Med Virol 2015;25:19–32 15. Morgan NG, Richardson SJ. Enteroviruses as causative agents in type 1 diabetes: loose ends or lost cause? Trends Endocrinol Metab 2014;25:611–619 16. Richer MJ, Horwitz MS. Coxsackievirus infection as an environmental factor in the etiology of type 1 diabetes. Autoimmun Rev 2009;8:611–615 17. Wu J, Shen L, Chen J, Xu H, Mao L. The role of microRNAs in enteroviral infections. Braz J Infect Dis 2015;19:510–516 18. Roggli E, Britan A, Gattesco S, et al. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes 2010; 59:978–986 19. Kim KW, Ho A, Alshabee-Akil A, et al. Coxsackievirus B5 infection induces dysregulation of microRNAs predicted to target known type 1 diabetes risk genes in human pancreatic islets. Diabetes 2016;65:996–1003 20. Laitinen OH, Honkanen H, Pakkanen O, et al. Coxsackievirus B1 is associated with induction of bcell autoimmunity that portends type 1 diabetes. Diabetes 2014;63:446–455 Figure 1—How a viral infection could impact on the development of autoimmunity by altering host miRNA expression. ① The enterovirus infects b-cells within an islet. ② The enterovirus replicates, and viral proteins are produced. ③ The virus induces upregulation and downregulation of specific host miRNAs. ④ miR-155-5p at early time points and miR-21-3p at later time points impact on the host cell apoptotic responses. ⑤ Several of the miRNAs impact on the expression levels of key pathogen recognition receptors (PRRs) and SOCS1, which could alter host response to infection. ⑥ miR-34a and miR-21-3p alter expression of key genes involved in the regulation of b-cell exocytosis (VAMP2, OC2), thus potentially impacting on host cell insulin secretion. ⑦ Furthermore, miRNAs can be packaged into microvesicles and/or endosomes and released from the infected cells. ⑧ Neighboring islet cells can take up secreted miRNAs, which can then subsequently impact on their gene expression, either making them more or less susceptible to infection or apoptosis, which can alter their insulin secretion. ⑨ Secreted miRNAs can also be taken up by infiltrating and peripheral immune cells, which could then significantly impact on their regulatory or effector cell functions.