* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nuclear Reactions - Socastee High School
Muon-catalyzed fusion wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear Reactions
Fission and Fusion
artificial (induced) transmutation
• Change of one element into another
through bombardment of the nucleus
– Nitrogen gas hit w/ alpha particles
(Rutherford, 1919)
• Determined to produce O & H (Blackett, 1925)
– O-16 hit w/ neutrons
nuclear reaction equations
• Written in the form of a chemical equation
• Balance mass numbers
• Balance atomic numbers
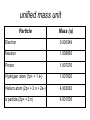
unified mass unit
•
•
•
•
Also called “Atomic Mass Unit”
Exactly 1/12th the mass of a carbon 12 atom
1 u = 1.661 10-27 kg
We need to be careful to distinguish between the
atomic mass and the nuclear mass.
– The atomic mass is the mass of an atom complete
with its electrons
– The nuclear mass is the mass of the nucleus alone.
To get the nuclear mass we need to take away the
mass of the electrons.
unified mass unit
Particle
Mass (u)
Electron
0.000549
Neutron
1.008665
Proton
1.007276
Hydrogen atom (1p+ + 1 e-)
1.007825
Helium atom (2p+ + 2 n + 2e-)
4.002063
α particle (2p+ + 2 n)
4.001505
Einstein’s mass-energy
equivalence relationship
• E = mc2
• Joules = Kilograms (m/s)2
• Used to calculate:
– Rest energy of a mass
• A mass not moving & has no additional energy due
to work done on it
– Energy released in nuclear reactions
Einstein’s mass-energy
equivalence relationship
•
•
•
•
Must convert the mass from u to kg
Energy calculated is in Joules.
Joules not convenient @ nuclear level
convert to electron volts (eV)
– divide by 1.6 10-19 J/eV
• A useful conversion factor between mass
and energy is that 1 u = 931.3 MeV
mass defect
• The total mass of the nucleus is less than
the sum of the mass of the individual
neutrons and protons that formed the
nucleus.
• The difference in mass is equivalent to the
energy released in forming the nucleus.
He mass defect
Particle
Mass (u)
Electron
0.000549
Neutron
1.008665
Proton
1.007276
#
2
2
2
Total (u)
0.001098
2.017330
2.014552
4.032980
• the atomic mass is 4.002603 u
• difference of 0.030377 u
• difference between the total mass of the
nucleons and the measured mass of the nucleus
mass defect
• So with our helium atom, the missing
0.030377 u is released when the nucleons
come together.
• That energy has to be put back to split the
nucleus up again.
binding energy
• neutrons and protons held together by 'strong
force'.
– acts over very small distances
– able to overcome the electrostatic repulsion between
protons
• most tightly bound nuclei are those close to iron
in the periodic table
• tightness of this binding is measured by the
binding energy per nucleon
• also sometimes called the mass defect per
nucleon
graph of binding energy
• The binding energy of atomic nuclei plotted against the
atomic number of the nuclei.
• Energy is released by the fusion of light elements into
heavier elements (elements on the left) or the fission of
heavy elements into lighter elements (elements on the
right).
graph of binding energy
From this graph we can see the following:
• The vast majority of nuclides have a binding energy of 8 MeV per
nucleon.
• Helium has a particularly high value of binding energy per nucleon,
much higher than the light isotopes of hydrogen.
• There is a trend for nuclides of nucleon numbers in multiples of 4 to
be particularly stable (i.e. have a high binding energy).
• Fe is the most stable nuclide.
• The largest nuclides tend to be less stable, with slightly lower
binding energies per nucleon.
graph of binding energy
• Higher = more stable
• Iron is the highest element on the graph, and the
most stable. It cannot release energy through
either fusion or fission.
graph of binding energy
• most tightly bound nuclei are those close to iron
in the periodic table of elements
• tightness of this binding is measured by the
binding energy per nucleon
• sometimes called the mass defect per nucleon.
graph of binding energy
• general decrease in binding energy beyond iron is
caused by the fact that, as the nucleus gets bigger, the
ability of the strong force to counteract the electrostatic
force between the protons becomes weaker
• peaks in binding energy at 4,8,16 and 24 nucleons is a
consequence of the great stability of helium-4 a
combination of two protons and two neutrons.
graph of binding energy
• The maximum binding energy at iron means that
elements lighter than iron release energy when
fused.
• This is the source of energy in stars and
hydrogen bombs.
graph of binding energy
• From the graph it can be seen that the greatest
release of energy occurs fusing hydrogen to
form helium.
graph of binding energy
• Elements heavier than iron only release energy
when split, as was the case with the plutonium
and uranium used in the first atomic bombs.
Sources of Heavy Elements
• Elements heavier than iron are made in
stars by capturing neutrons onto atomic
nuclei.
• This takes place in some red giants and in
supernovae explosions.
Sources of Heavy Elements
• A new isotope is created when an atom captures a
neutron.
• If this isotope is unstable then a neutron can convert into
a proton, releasing an electron.
• This is called beta decay and is a form of radioactive
decay also observed on Earth.
• By converting a neutron into a proton the atom has
increased its atomic number by one and become the
adjacent element in the periodic table.
• It may then capture another neutron, and so on, so that
using iron as seed nuclei it is possible to build all the
elements heavier than iron in the periodic table.
Sources of Heavy Elements
• The difference between element synthesis
in red giants and supernovae is that in
supernovae the flux of neutrons is greater
and it is possible for the atom to capture a
second, or third neutron, before it has a
chance to beta-decay.
• This leads to the production of a different
set of elements to those produced in red
giants, where the flux of neutrons is much
less.
graph of binding energy
• Radioactive decay happens when an unstable
nucleus emits radiation.
• It becomes more stable.
• The daughter nuclei always have a higher
binding energy per nucleon than the parent
nucleus
nuclear fission
• The large nuclei have a lower binding
energy per nucleon.
• They are less stable.
• This lack of stability is usually shown by
radioactive decay, which occurs in a
predictable way. Very rarely a large
nucleus will split up spontaneously into
fragments.
• Splitting of the nucleus is called fission.
nuclear fission
• Consider the nucleus as a
“wobbly drop”.
• Nuclei are not tidy and neatly
arranged rows of neutrons
and protons.
• They are microscopic
bedlam.
• The strong nuclear force acts
between neighboring
nucleons
nuclear fission
• The nucleons are not linked with the same
neighbors all the time. Instead they are
constantly swapping about. However the
enough of the nucleons linked to stop the
repulsive electromagnetic force tearing the
nucleus apart.
• Now we imagine the nucleus as a wobbly drop:
nuclear fission
• Now if the nucleus gets to this shape:
nuclear fission
• The nucleus flies apart in two fragments:
The detail of the mechanism that drives this process is
complicated and is based on Heisenberg’s uncertainty
principle. A similar model can be used to explain how
alpha decay works.
nuclear fission
• We can induce fission in large nuclei such as
uranium-235. The most common isotope of
uranium, U-238, does not split easily, but the
235 isotope does. We induce fission by “tickling”
the nucleus with a “thermal” neutron. The
neutron has to have the right kinetic energy:
– Too little kinetic energy means that the neutron will
bounce off the nucleus;
– Too much kinetic energy means that the neutron will
go right through the nucleus.
– Just right means that the neutron will be captured by
the strong force, which is attractive between
nucleons. The neutron gives the nucleus enough
energy to resonate, and this will make the nucleus
neck as shown previously
nuclear fission
• The tickled nucleus flies apart into a number of
fragments, leaving on average three neutrons
left over.
• These too are able to tickle other nuclei and
make them split.
• Each neutron spawns three more neutrons in
each fission, so we get a chain reaction.
nuclear fission
• There is a mass defect in the products of
the fission so energy is given out.
• In an uncontrolled chain reaction, the
energy is given out in the form of a violent
explosion, which is many times more
powerful than the explosive decomposition
of TNT.
• In an atomic bomb, the mass that is
converted to energy is about 20 grams.
nuclear fusion
•
•
•
•
Light nuclei are joined together
Increases the binding energy per nucleon
Results in lots of energy being given out
An example:
nuclear fusion
• It is not simply a case of sticking some
deuterium and tritium together and shaking it up.
Each nucleus has to have sufficient energy to:
– Overcome electrostatic repulsion from the protons;
– Overcome the repulsive strong force which is found
outside the region of the strong force.
• This means that the gases have to be heated to
a very high temperature, 100 million Kelvin.
nuclear fusion
• main source of the Sun’s energy
problems involving fission and
fusion reactions
•
•
•
•
•
Data to use:
Mass of deuterium nucleus = 3.3425 10-27 kg
Mass of tritium nucleus = 6.6425 10-27 kg
Mass of helium nucleus = 6.6465 10-27 kg
Mass of a neutron = 1.675 10-27 kg
• What is the energy in J and eV released in this
reaction above?
URANIUM, PLUTONIUM, & THE
BOMB
• When uranium ore is extracted from the
earth, most of the uranium is removed
from the crushed rock during the
milling process, but the radioactive
decay products are left in the tailings.
Thus 85 percent of the radioactivity of
the original ore is discarded in the mill
tailings.
URANIUM, PLUTONIUM, & THE
BOMB
• Depleted uranium remains radioactive
for literally billions of years, and over
these long periods of time it will
continue to produce all of its
radioactive decay products; thus
depleted uranium actually becomes
more radioactive as the centuries and
millennia go by because these decay
products accumulate.
URANIUM, PLUTONIUM, & THE
BOMB
• Natural uranium is a
blend of two types : U235 and U-238.
• At a uranium enrichment
plant, the concentration
of U-235 is increased by
discarding some U-238.
• The cast-off uranium
(mainly U-238), called
''depleted uranium'', has
virtually no commercial
value.
URANIUM, PLUTONIUM, & THE
BOMB
There are several important military
uses for depleted uranium :
1.
2.
3.
4.
when placed in a reactor, it breeds plutonium -- a
powerful nuclear explosive;
when incorporated into an H-bomb, it doubles the
explosive power of the weapon;
when used to coat conventional bullets and shells,
it makes them armour-piercing;
when used as a metallic alloy in tanks and other
vehicles, it provides armour-plating.
Breeding plutonium-239 from
uranium-238
How To Make an H-Bomb
Howard Morland wrote a
magazine article
explaining how an "HBomb" -- or
"thermonuclear bomb" -- is
made, using only publicly
available information. In
the photo, he is standing
on the steps of the US
Supreme Court holding a
cut-away model of the Hbomb.
How To Make an H-Bomb
• An H-bomb is a three-stage
weapon: fission, fusion, and
then fission again. The first
stage, called the "trigger"
(the black ball at the top), is a
small plutonium bomb
similar to the one dropped
on Nagasaki in 1945. The
energy release at this stage
is mainly due to nuclear
fission -- because the atoms
of plutonium are split.
How To Make an H-Bomb
• Tritium is often added to the
centre of the plutonium core
to "boost" the fission
explosion with some
additional fusion energy.
Boosted or not, however, the
only importance of this firststage explosion is to
irradiate and heat the
material in the central
column to 100 million
degrees celsius so that a
much more powerful fusion
reaction can be started there.
How To Make an H-Bomb
• The second stage explosion is due
to nuclear fusion in the central
column. The main fusion reaction
involves concentrated deuterium
and tritium (both heavy isotopes of
hydrogen) -- which become
spontaneously available when
neutrons from the first stage
explosion bombard a solid material
called "lithium deuteride" located
in the central column. When this
hydrogen-rich mix is heated to 100
million degrees, the deuterium and
tritium atoms "fuse" together,
releasing enormous amounts of
energy. This is the "H" or
"thermonuclear" part of the bomb.
How To Make an H-Bomb
• Then comes the third stage.
The fusion reaction gives off
an incredible burst of
extremely powerful neutrons
-- so powerful that they can
split or "fission" atoms of
uranium-238 (called
"depleted uranium") -- which
is impossible at lower energy
levels. This third stage more
than doubles the power of
the explosion, and produces
most of the radioactive
fallout from the weapon.
How To Make an H-Bomb
• Unlike fission bombs, which rely
only on nuclear fission, and
which can achieve explosions
equivalent to thousands of tons
of TNT ("kilotons"), the power of
an H-bomb or thermonuclear
weapon has no practical limit -it can be made as powerful as
you want, by adding more
deuterium/tritium to the second
stage. Most H-bombs are
measured in "megatons"
(equivalent to the explosive
power of MILLIONS of tons of
TNT -- hundreds of times, or
even a thousand times more
powerful than a fission bomb).