* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hypertensive anterior uveitis
Immune system wikipedia , lookup
Adaptive immune system wikipedia , lookup
DNA vaccination wikipedia , lookup
Molecular mimicry wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Hepatitis B wikipedia , lookup
Behçet's disease wikipedia , lookup
Innate immune system wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
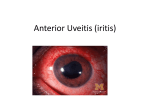
Staff Lecture 2015.4.15 Uveitic Glaucoma Hypertensive anterior uveitis 성빈센트병원 안과 최진아 Glaucoma associated with Ocular inflammation • Clinical course – Acute – Subacute – Chronic • Etiology - Infectious - Non-infectious • Location – – – – Anterior 45.8 % Intermediate 15.3 % posterior 14.5 % Panuveitis (anterior and posterior) 24.5% Non-infectious uveitic condition Infectious uveitic condition • Bacterial and fungal disease • AIDS • Acute retinal necrosis and progressive outer retinal necrosis • Other viral disease – HSV keratitis and keratouveitis – H.zoster ophthalmicus • • • • Ocular toxoplasmosis Ocular histoplasmosis Toxocara canis Post-surgical endophthalmitis • Anterior uveitis – Idiopathic – HLA-B27 associated – HLA-B27 associated with systemic disease • • • • • • • • • • • Scleritis Intermediate uveitis Sarcoidosis Sympathetic ophthalmia Vogt-Koyaagi-Harada disease Birdshot Retinochoroidopathy Behcet’s disease Retinal vasculitis Serpiginous Choroidopathy White-dot syndrome Marsquarade syndrome Anterior uveitis • The most common form of uveitis • Associated complications such as glaucoma may result in severe visual loss • Major indicator – Presence of cells and flare in the anterior chamber • Category – Irititis (inflammation of the iris) – Iridocyclitis (inflammation of the iris and ciliary body) • The form of ocular inflammation producing the elevation of intraocular pressure is iridocyclitis. Aqueous production • Inflammation of the ciliary body – Reduced aqueous production Aqueous Outflow • Acute mechanism • – Open angle • Inflammatory cells and fibrin • Prostaglandin • Swelling or dysfunction of the trabecular lamellae or endothelium • Precipitates on the trabecular meshwork • Use of corticosteroid – Closed angle • Acute pupillary block glaucoma • Uveal effusion with forward rotation of the ciliary body • Displacement of the lens-iris diaphragm due to exudative retinal detachment Chronic mechanism – Scarring and obliteration of outflow channel – Overgrowth of fibrovascular membrane – Synechial closure of the angle – Posterior synechiae and iris bombe Syndromes of Anterior Uveitis Uveitis, Mosby Disease Age Sex Ocular Redness HLAB27 Steroid response Systemic finding Idiopathic Any Either Yes No Yes None HLA-B27, ocular only 15-40 M>F Yes Yes Yes None Ankylosing spondylitis 15-40 M>F Yes Yes Yes Spondylitis, sacroiliitis Reiter’s syndrome 15-40 M>F Yes Yes Yes Arthritis, urethritis, mucocutaneous lesion Juvenile rheumatoid arthritis 3-16 F>M No No Yes Pauciarticular arthritis Fuchs’ iridocyclitis Any Either No No Yes / No None PosnerSchlossman syndrome Adult Either No No Yes None Schwartz syndrome Adult Either No No 1. Onset of IOP elevation 2. Correlation between inflammation Noand IOP elevation None Ocular ischemia >50 Either Yes No No Carotid insufficiency Kawasaki disease 1-18 Either Yes No Yes Skin rash.. Glaucomatocyclitic crisis : Posner-Schlossmann syndrome • Uniocular, young to middle aged adult • Recurrent attack of mild uveitis with marked elevation of IOP ranged of 40-60 mmHg • Between attack : IOP and facility of aqueous outflow usually normal Fuchs Heterochromic Cyclitis • Mild uveitis, heterochromia, cataract, occasional glaucoma • Uveitis runs a single, very protracted course • IOP elevation occur as a late, serious complication Case I HAU A 27 year-old female with ocular discomfort (OS) History of Posner-Schlossman syndrome for several years IOP: 36 mm Hg • On low magnification slit lamp examination, the cornea showed some fine keratic precipitates. • On high magnification slit lamp examination, fine hammered silverappearance of entire corneal endothelium was seen. Choi JA, La TY, International Journal of Ophthalmology, 2014 Case I HAU Case 2 HAU A 53-year-old male with suddenly decreased VA(OD) BCVA: 0.15, IOP: 55mmHg Preoperative images: The slit lamp exam shows (A) Fine round or stellate keratic precipitates and fine filaments on the endothelium between the keratic precipitates (B) Patchy loss of the iris pigment epithelium. Lee JA and Choi JA, KJO, in Press Case 2 HAU After the Ahmed valve implantation (A) The valve tip was well positioned at superotemporal side (POD 1d) (B) The slit lamp shows depigmented atrophic iris and posterior synechiae(POD 8m) (C) Before PPKP Case 2 HAU Remarkable changes of corneal endothelial cell density: (A) Before Ahmed valve implantation (B) POD 7 months after Ahmed valve implantation (C) POD 13 months after Ahmed valve implantation (D) Before cataract surgery Hypertensive Anterior Uveitis - associated with virus I. Clinical Characteristics : CMV Corneal endothelium II. Etiology : T-cell senescence vs. lymphopenia 1. Clinical characteristics of HAU Emerging viral anterior uveitis • A cause of what previously had been considered to be idiopathic ocular inflammations • Acute, recurrent and chronic anterior uveitis associated with IOP elevation • Three members of the lifelong Herpesviridae family – herpes simplex virus (HSV) – varicella zoster virus (VZV) – most recently, cytomegalovirus (CMV) 1. Clinical characteristics of HAU I. HSV-associated anterior uveitis • Recurrent conjunctivitis, keratitis, and uveitis • 28% had IOP elevation and 10% had glaucomatous damage in patients with HSV keratouveitis. • Disciform or stromal form keratitis – More associated with IOP elevation and keratitic precipitate 1. Clinical characteristics of HAU II. VZV-associated anterior uveitis • Characteristic cutaneous vesicular eruption along the trigemimal distribution • Often occurs with herpes zoster ophthalmicus • Keratitis and uveitis • Commonly leads to glaucoma • Sectorial iris atrophy • Mutton fat keratic precipitates 1. Clinical characteristics of HAU III. CMV-associated anterior uveitis • the pathogen most recently implicated in anterior uveitis • acute, recurrent or chronic disease. • present as acute relapsing hypertensive anterior uveitis – – – – Posner-Schlossman syndrome (PSS) Fuchs’ heterochromic iridocyclitis (FHI) Corneal endotheliitis Sector iris atrophy with iritis • Often fail to respond to corticosteroid 1. Clinical characteristics of CMV-HAU Typical feature of CMV anterior uveitis Acute form Chronic form • Episodic hypertensive anterior uveitis • resembling the PosnerSchlossmann syndrome, with attacks of mild iritis, elevated IOP and diffuse corneal epithelial edema, and a few fine KPs. • Although the IOP is normal in between attacks and the angles are open, about 23% of these eyes may develop glaucomatous damage as a result of the repeated attacks. • a chronic variety of CMVassociated anterior uveitis • manifests in a manner similar to Fuchs heterochromic iridocyclitis • (FHI) with asymptomatic, mild inflammation, and diffuse stellate KPs. • There may be diffuse iris atrophy. • Posterior synechiae are typically • absent. • The IOP is often elevated and glaucomatous optic atrophy is seen in 36%. Corneal endotheliitis • white, medium sized, nodular deposits were also noted on the endothelium • a surrounding translucent halo • range from a small localized area to diffuse bullous keratopathy • In eyes with focal edema, a sharp demarcation line may be present • The KPs are usually seen in the inferior half of the cornea and may be diffuse, linear, or organized in a ring pattern or appear as a coinlike lesion. • In Singapore, CMV was found to be the infective agent in 22.8% of eyes with hypertensive anterior uveitis and in all the cases of corneal endotheliitis. Ophthalmology 2007;114:798–803 Am J Ophthalmol 2012;153:445–453 1. Clinical characteristics of CMV-HAU Association of viral load with IOP elevation Jpn J Ophthalmol 2013:57:497–502 Graefes Arch Clin Exp Ophthalmol 2014:252:117–124 1. Clinical characteristics of CMV-HAU Association of viral load with corneal endothelial cell loss • Correlation between cytomegalovirus (CMV) viral load and corneal endothelial cell damage (r=0.664; p=0.036) Br J Ophthalmol 2010;94:336e340 Jpn J Ophthalmol 2013:57:497–502 1. Clinical characteristics of CMV-HAU • Mean patient age : 66.9±10.9 yrs • 85 males (80.2%), 21 females (19.8%) • Patients were commonly diagnosed with anterior uveitis and ocular hypertension prior to confirmation of CMV endotheliitis. • Coin-shaped lesions were observed in 70.6%, and linear keratic precipitates in 8.3% of the patients, respectively. Br J Ophthalmol. 2015;99(1):54-8. 1. Clinical characteristics of CMV-HAU Treatment of CMV anterior uveitis (1) • Ganciclovir – first phosphorylated by the viral kinase UL97, – further phosphorylated by cellular kinases to Ganciclovir triphosphate – inhibits the viral DNA polymerase UL54 – Systemic form – Intravitreal – Topical form (gel) – Side effect : neutropenia, thrombocytopenia, anemia, renal dysfunction, confusion, nausea, and thrombophlebitis 1. Clinical characteristics of CMV-HAU Treatment of CMV anterior uveitis (2) • Valganciclovir – a valine ester of Ganciclovir, which has much better oral bioavailability than Ganciclovir • Foscarnet – Side effect : nephrotoxicity, electrolyte imbalance, nausea, vomiting, penile ulceration, headache, seizure, thrombophlebitis • Cidofovir – Seldom used 1. Clinical characteristics of CMV-HAU Treatment of CMV anterior uveitis (3) • All virostatic and no virucidal • In majority of the cases, the inflammation resolves with therapy. • Inflammation frequently recurs after the anti-CMV treatment is stopped. • The optimal modality as well as duration of therapy has yet to be determined for non-HIV patients with anterior segment disease. 1. Clinical characteristics of CMV-HAU Br J Ophthalmol. 2015;99(1):54-8. Br J Ophthalmol 2010;94:1648e1652 Graefes Arch Clin Exp Ophthalmol 2014; 252:117–124 1. Clinical characteristics of CMV-HAU Prognosis of CMV anterior uveitis • 36.4% of patients required surgical treatment in addition to oral valganciclovir administration to stabilize the IOP in CMV-related Posner- Schlossman syndrome. Graefes Arch Clin Exp Ophthalmol 2014;252(1):117–124. Patients with CMV-positive eyes with a disease duration over 5 years were likely to require glaucoma surgery (P =0.024, log-rank test). Am J Ophthalmol 2014;158:1024–1031 1. Clinical characteristics of CMV-HAU Cytomegalovirus as a cause of acute endothelial cell loss in immunocompetent patients with hypertensive anterior uveitis 1.Clinical Clinicalcharacteristics characteristicsofofHA CMV-HAU Method • Seoul St. Mary’s Hospital from March 2009 to June 2014 • Clinical comparative study design • Inclusion criteria – (1) mild anterior uveitis with keratic precipitates (KPs) – (2) increased intraocular pressure (IOP). • Exclusion criteria – (1) presence of inflammation in vitreous or retina; and (2) presence of corneal changes for a known cause; for example, those with corneal dystrophies. 1. Clinical characteristics of CMV-HAU • Aqueous sampling – Using a 30-gauge needle, 100 µL aqueous humor was aspirated under aseptic conditions and subjected to a polymerase chain reaction (PCR) assay for CMV and HSV DNA. • PCR – DNA was extracted from the aqueous humor samples using a QIAamp DNA minikit (Qiagen, Valencia, CA, USA). – Quantitative CMV-DNA PCR testing was performed using an AccuPower CMV Quantitative PCR Kit (Bioneer, Daejun, Republic of Korea). – For HSV PCR, the HSV 1/2 PCR Kit (Bio-Core, Seoul, Republic of Korea) was used. 1. Clinical characteristics of HAU Clinical parameters of 42 patients with hypertensive anterior uveitis Gender (M:F) 29:13 Age (range), years 57.6 (25–88) Spherical equivalent, D -2.6 (-11.25–0.00) Glaucoma operation,% 22 (52.4%) Corneal endothelial cell count, cells/mm2 1,908 (625–3067) Unilaterality 39 (92.9%) KPs at baseline examination 28 (67.8%) Anterior chamber reaction with 1+ or less 37 (88.1%) Severe peripheral anterior synechiae 0 (0.0%) Typical feature of P-S syndrome 14 (33.3%) Comparisons ofClinical Clinical and Immunologic Characteristics in Comparisons of and Immunologic Characteristics in Subjects with without CMV-PCR (+) in Aqueous Subjects with or or without CMV-PCR (+) in Aqueous Humor Humor Characteristics CMV-positive Subjects CMV-negative subjects P value n=6 n = 16 Male, n(%) 6 (100%) 13 (86.7%) 0.500 Age, years 47.5 ± 14.8 67.6 ± 11.8 0.006 Initial BCVA 0.65 ± 0.29 0.58 ± 0.29 0.569 Final BCVA 0.47 ± 0.46 0.52 ± 0.30 0.733 Spherical equivalent, D -3.6 ± 4.2 0.0 ± 1.6 0.031 Axial length, mm 25.7 ± 1.5 24.4 ± 0.7 0.053 Average RNFL thickness, µm 66.2 ± 16.7 77.8 ± 19.1 0.132 Corneal endothelial cell count, mm2 1245 ± 560 1981 ± 387 0.009 Unilaterality, n(%) 83.3 80.0 0.684 KPs at baseline examination, n(%) 83.3 73.3 0.550 4 (66.7%) 5 (33.3%) 0.331 100.0 92.9 0.714 Demographic characteristics Ocular characteristics Presence of PAS, n(%) Anterior chamber reaction with < 1+, n(%) Characteristics CMV-positive CMV-negative subjects subjects 5 (83.3%) 5 (33.3%) 0.055 Baseline IOP, mmHg 28.83 ± 8.25 22.73 ± 7.86 0.132 Maximum IOP, mmHg 37.17 ± 8.03 39.53 ± 13.02 0.622 2.67 ± 0.81 2.67 ± 0.81 0.970 Chronic , n(%) 4 (66.7%) 13 (86.7%) 0.544 Recurrent, n(%) 2 (33.3%) 2 (13.3%) 4 (66.7%) 10 (66.7%) 0.701 Ahmed valve, n(%) 3 (75.0%) 2 (20.0%) 0.095 Trabeculectomy or ExPRESS glaucoma filtration 1 (25.0%) 8 (80.0%) HSV PCR positivity, n(%) 0 (0.0%) 1 (6.2%) 0.727 CMV RQ PCR, copies/mL 46048.8 ± 91334.5 Negative n/a Lens status, phakic,, n(%) Number of antiglaucoma medication, n P value Course of uveitis Glaucoma operation, n(%) device, n(%) 1. Clinical characteristics of HAU Association between presence in aqueous Association between thethe presence CMV CMV DNA inDNA aqueous and corneal endothelial cell count patients with anterior and corneal endothelial cell in count in patients with anterior hypertensive uveitishypertensive uveitis Model 1 Model 2 Model 3 Adjusted for age, gender, Adjusted for age and Unadjusted lens status, and history of gender glaucoma surgery CMV positivity P value B (CI) B (CI) B (CI) 672.4 734.4 1034.2 (77.0–1267.9) (116.4–1352.4) (631.3–1437.1) 0.029 0.023 < 0.001 1. Clinical characteristics of HAU In CMV-associated uveitis, extensive corneal endothelial cell damage may occur. Hypertensive Anterior Uveitis - associated with virus I. Clinical Characteristics : CMV Corneal endothelium II. Etiology : T-cell senescence vs. lymphopenia 2. Etiology of CMV-HAU Latency of CMV • HCMV establishes lifelong latency after the primary infection. – a reversibly quiescent state in which viral genomes are maintained, but viral gene expression is highly restricted and no virus is produced • Seropositivity – In industrialized countries, such as Australia and the United States, seroprevalence of CMV is about 60% – In less developed regions it is about 90%. • the primary sites of HCMV latency – CD34+ myeloid progenitor cells • non-permissive • specific human cellular DNA-binding proteins in the nucleus bind to the CMV immediate-early (IE) promoter and inhibit transcription, thereby blocking the production of infectious particles. 2. Etiology of CMV-HAU CMV Reactivation from Latency • As the latently infected myeloid progenitor cells divide and differentiate into monocytes, the virus is carried out of the bone marrow in circulating monocytes and subsequently in tissue macrophages and dendritic cells throughout the body. • When latently infected macrophages and dendritic cells subsequently become highly activated, the conditions inside the nucleus change. • The human cellular DNA binding proteins that previously bound to the CMV immediate-early promoter disappear, permitting the virus to reactivate from latency. 2. Etiology of CMV-HAU The host immune response against CMV • B cell response – In response to primary CMV infection, anti-CMV antibody is produced initially as IgM followed by IgG, which persists lifelong. • T cell response – The most important immune responses against CMV are CD4+ T cells and CD8+T cells. – patients who have severely impaired T-cell responses can develop serious CMV disease despite having anti-CMV antibody • eg, solid organ transplantation, allogeneic bone marrow transplantation • in advanced HIV infection, immunosuppressive treatment 2. Etiology of CMV-HAU Viral Escape from host immune system • In infected cells, the virus can produce over 250 proteins, but only about 50–60 are believed to be essential for viral replication. – Thus, the vast majority of these viral proteins enable the virus to coexist with its host. • to facilitate viral production • to avoid detection and elimination of the virus by the immune system. • CMV immune evasion genes – interfere with the MHC Class I antigen presentation pathway 2. Etiology of CMV-HAU Questions WHY DOES CMV REACTIVATE IN ANTERIOR CHAMBER OF “IMMUNOCOMPETENT”? ARE THEY REALLY “IMMUNOCOMPETENT”? 2. Etiology of CMV-HAU Question 1 WHY DOES CMV REACTIVATE IN ANTERIOR CHAMBER OF “IMMUNOCOMPETENT”? 2. Etiology of CMV-HAU I. Chronic inflammation CMV reactivation in inflamed tissue • HCMV may be reactivated when these cells are recruited to sites of inflammation. -In vitro, latent virus in macrophages can be reactivated by allogeneic stimulation of peripheral blood mononuclear cells or by differentiation into dendritic cells. -Inflammatory cytokines such as tumor necrosis factor (TNF) , interferon (IFN) and granuocytemonocyte colony-stimulating factor seem to be important in this process. Chronic inflammation as a trigger for CMV reactivation in anterior chamber? 2. Etiology of CMV-HAU II. Ocular immune privilege • Direct venous drainage of aqueous humor – Lack of lymphatic drainage • Anterior –chamber associated immune deviation – eye-derived CD1+ APCs – Induction of the antigen-specific regulatory T cells mediate ACAID emerge from the cell clusters • CD4+ Treg cell (afferent) • CD8+ Treg cells (efferent) 2. Etiology of CMV-HAU • Intraocular immunosuppressive microenvironment TGF beta T cell : inhibit proliferation and effector functions upregulate the Treg B cells: inhibit proliferation, IgA production Macrophage: inhibit proliferation and prevent the production of reactive oxygen and nitrogen. Acts as an chemoattractant Fibroblast : increased collagen synthesis 2. Etiology of CMV-HAU III. Chronic topical steroid use • Immunosuppression by steroid – mainly achieved by the derangement of primarily T lymphocyte and monocyte/macrophage functions • inhibits the function of NF-κB (IκB gene) • NF-kB – a key activator of proinflammatory cytokines – an important transcription factor involved in T cell activation • diminish the transcription of IL-1 and TNF-α by APCs • prevent the upregulation of MHC expression • Phospholipase A2, and consequently the entire arachidonic acid cascade, is also inhibited. • They decrease the leukocyte response to various chemokines and chemotactins; by inhibiting vasodilators such as histamine and prostacyclin 2. Etiology of CMV-HAU III. Chronic topical steroid use Steroid inhibits the function of NF-κB (IκB gene) Sabiston Textbook of surgery Cytomegalovirus endotheliitis after fluocinolone acetonide (Retisert) implant in a patient with Behçet uveitis • A 40-year-old man received a Retisert implant in the left eye for recurrent Behcet uveitis. • PCR for aqueous humor detected 3.9 × 104 copies/mL of CMV DNA. Ocul Immunol Inflamm 2011;19(4):282-3 Cytomegalovirus Retinitis After Intravitreous Triamcinolone Injection in a Patient with Central Retinal Vein Occlusion A 77‐year‐old woman with macular edema due to central retinal vein occlusion (CRVO) developed peripheral retinitis 4 months after IVTA. Korean J Ophthalmol. 2008;22(2):143-4 Chronic ocular inflammation Chronic steroid Ocular immune use privilege Persistent viral activation even in immunocompetent? 2. Etiology of CMV-HAU Question 2 ARE THEY REALLY “IMMUNOCOMPETENT”? 2. Etiology of CMV-HAU Immunosenescence (Immune aging) • The gradual deterioration of the immune system brought on by natural age advancement. • increased frequency of morbidity and mortality among the elderly. • T-cell functional dysregulation as a biomarker for immunosenescence Immune Risk Phenotype 2. Etiology of CMV-HAU • Multiple latent persistent viral infections, including HSV, varicella-zoster virus, EBV, and, above all, CMV. • Human CMV appears to be the most immunodominant antigen encountered by the immune system throughout the life of most individuals. • The response against HCMV mediated by CD8+ T cells in healthy elderly persons may constitute as much as 50% of the entire CD8+ repertoire. 2. Etiology of CMV-HAU • Memory inflation of the antiviral T cell population – repeated interactions between CMV and Ag-specific T cells, which can occupy up to 50% of the human T cell pool in late life – Due to CMV’s exquisite immune evasion and reactivation properties. • Gradual accumulation of late-differentiated, antigenspecific, oligoclonal T cells, particularly within the CD8+ T-cell compartment – critically shortened telomeres – loss of CD28 and/or gain of CD57 expression – defined as CD8+CD28- or CD8+CD57+ T lymphocytes 2. Etiology of CMV-HAU Immunity & Ageing 2012, 9:23 Age and CMV infection are major driving forces contributing to the deterioration of innate and adaptive immunity 2. Etiology of CMV-HAU Immunologic status of patients with hypertensive anterior uveitis 2. Etiology of CMV-HAU Method • 2014.3~ 2015.2 St. Vincent’s Hospital • Prospective study design • Hypertensive Anterior Uveitis – Inclusion criteria • (1) anterior uveitis with keratic precipitates (KPs) • (2) increased intraocular pressure (IOP). – Exclusion criteria • (1) presence of inflammation in vitreous or retina • (2) presence of corneal changes for a known cause; for example, those with corneal dystrophies. • Controls – Cataract patients – No sign of uveitis – No episode of increased IOP • Leukocyte subset frequency determination – PBMC (peripheral blood mononuclear cells) • Obtained by density gradient centrifugation – FACS • • • • • CD57 FITC (IM0466U) CD28 PE (IM2071U) CD4 PE – CY 5.5 (9522-16) CD8 PE – CY 7 (9536-17) All reagents from Beckman Coulter, Inc. • Serum cytokine level determination – Cytokines were measured using a Luminex system (Luminex Corp). – IFN-γ, IL-2, IL-6 – TGF-β1,2,3 2. Etiology of CMV-HAU Comparison of Clinical Parameters between patients with hypertensive anterior uveitis and controls HAU Control 15 12 61.2 ± 11.6 63.4 ± 7.2 0.590 6 (46.1%) 4 (36.4%) 0.697 CMV IgG titer, AU/mL 208.2 ± 55.8 168.4 ± 73.8 0.303 HSV IgG titer, AU/mL 6.6 ± 2.3 8.0 ± 0.0 0.119 2098.1 ± 650.2 2796.9 ± 333.2 0.001 n Age, yrs Gender, % of female Corneal endothelial cell count, cells/mm2 P value HAU Controls 2. Etiology of CMV-HAU Acute hematologic effects of interferon alpha, interferon gamma, tumor necrosis factor alpha and Interleukin 2 Ann Hematol (1991) 62:25-31 2. Etiology of CMV-HAU Peripheral Blood Lymphopenia due to lymphocyte homing • A recent infection • viral, bacterial, and fungal agents (m/c) • Idiopathic CD4+ lymphocytopenia • Corticosteroid use • Malnutrition • Systemic lupus erythematosus • Severe stress • Intense or prolonged physical exercise • Rheumatoid arthritis • Sarcoidosis • Malignancies leukemia or advanced Hodgkin's disease • Iatrogenic conditions • Chemotherapy • Large doses of radiation • Characterized by a transient reduction of peripheral blood lymphocyte counts, a phenomenon called “lymphopenia” • Induced by the redistribution of circulating lymphocytes from the blood to other lymphoid compartments (homing), resulting in the inability to access target organs. 2. Etiology of CMV-HAU Peripheral Blood Lymphopenia due to lymphocyte homing • Pre-transplant Lymphopenia (absolute lymphopenia < 500 cells/mm3) Is a Novel Prognostic Factor in CMV and non-CMV Invasive Infections After Liver Transplantation. Liver transplantation 2014;20(12):1497 • Lymphopenia, presence of symptoms and other infections and older age are significant risk factors for a poor outcome for CMV infection in rheumatic disease. Rheumatology 2008;47:1373 2. Etiology of CMV-HAU Aging vs. CMV effect on immune function • Aging – decreased immune cell function – lower antibody responses to influenza vaccination • CMV effect in young adults – young CMV-infected adults – an overall up-regulation of immune function – Enhanced antibody responses to influenza vaccination – Increased CD8+ T cell sensitivity – Elevated levels of circulating IFN-γ compared to uninfected individuals Sci Transl Med. 2015 Apr 1;7(281):281ra43. Summary in CMV-associated hypertensive anterior uveitis – CMV was a significant etiological factor in hypertensive anterior uveitis patients in Korea. – In CMV-associated uveitis, extensive corneal endothelial cell damage may occur. • Special caution is needed for patients with CMV-positive hypertensive anterior uveitis, given its adverse effects on the corneal endothelium. – Patients with hypertensive anterior uveitis do not appears to accompany typical immune aging. • The mechanism of hypertensive anterior uveitis seems to be related with systemic immune activation and resultant relative lymphopenia, rather than T-cell senescence. 경청해 주셔서 감사합니다