* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Correlation of Endothelial Cell Shape and Wall Shear Stress in a
Survey
Document related concepts
Transcript
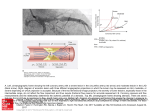
Correlation of Endothelial Cell Shape and Wall Shear Stress in a Stenosed Dog Aorta Murina J. Levesque, Dieter Liepsch, Stafan Moravec, and Robert M. Nerem Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 The pattern of endothelial geometry at various locations along stenosed dog aortas was examined. This was done to test the hypotheses that the shape of an endothelial cell is related to the local wall shear stress associated with the flowing blood and that alterations in hemodynamics, produced by vascular geometrical changes, influence endothelial cell geometry. Aortic stenosis with a reduction of 7 1 % of the cross-sectional area was produced. The animals were sacrificed 12 weeks later, and the endothelial cell geometry and orientation were studied using the vascular casting technique and a computerized analysis to determine cell area and shape index. The regions of the stenosis examined were those known to experience different hemodynamic conditions. The value of the shape index was found to fall rapidly in the convergent region of the stenosis and to increase suddenly in the divergent region, eventually returning to the prestenotic value at a more distal site. Using a model of a stenosis made from a vascular cast, laser Doppler anemometry was applied to measure velocity profiles and to estimate the local wall shear stress in a stenosed aorta. It is shown that the shape index distribution along these stenosed vessels may be correlated with the level of wall shear stress, with more elongated cells occurring in regions of higher shear stress. (Arteriosclerosis 6:220-229, March/April 1986) T forces, in particular arterial wall shear stress, stems from the belief that it is through this hemodynamically imposed frictional force that a fluid mechanic effect on the endothelium becomes manifest. In this context, the topic of interest is the influence of hemodynamic forces on endothelial morphology, cell function, cell turnover rate, and transendothelial transport, and it is the first of these, endothelial morphology, more specifically, en face cell shape, which is the subject of this investigation. There is a body of accumulating data that indicates that endothelial cell geometry and orientation are determined by hemodynamic forces. In vivo, Flaherty et al. 6 demonstrated that the orientation of endothelial cell nuclei (and thus by implication the orientation of the endothelial cells themselves) is determined by the hemodynamic stress associated with the flow of blood. Silkworth and Stehbens7 studied qualitatively the shape of endothelial cells using the en face Hautchen technique. They found that endothelial cells tended to align themselves with the flow field, and had an elongated tear-drop configuration, with the cell nucleus and the bulk of the cell cytoplasm distal to the midpoint of the longitudinal axis of the cell. In addition, endothelial cells about orifices of branches were often polygonal in outline. These investigators thus concluded that the shape and orientation of endothelial cells are influenced by the flowing blood and that changes from a true spindle shape could be the consequence of shear stresses. In our own work, we have conducted quantitative studies8' 9 on the morphology of aortic endothelium using the new technique of vascular casting that was developed by Reidy and Levesque.10 Using this vascular casting technique,11 we have found that in uniform segments of rabbit he evidence for the involvement of fluid dynamics in the atherosclerotic process centers primarily on the pattern of the disease.1"3 It is often regions of arterial branching and sharp curvature that have the greatest predilection for the development of atherosclerosis. These are also regions where the flow will assume unusual characteristics or at least deviate from what otherwise might be considered a well-behaved arterial flow. The indictment provided by this indirect evidence, particularly as it relates to the bifurcations and geometrical contortions of the arterial vasculature, presently motivates much of the interest in arterial fluid dynamics. It has been suggested that vascular geometry may affect the atherogenic process through its influence on the hemodynamic environment to which the intima is exposed.4' 5 Under such a hypothesis, whatever affects vascular geometry would alter the local detailed flow characteristics and correspondingly influence endothelial morphology and function. Our interest in hemodynamic From the Physiological Fluid Mechanics Laboratory, Department of Mechanical Engineering, University of Houston, Houston, Texas and the Fachbereich Versorgungstechnik, Fachhochschule, Munich, Federal Republic of Germany. This research was supported by Grant MEA-8200344 from the National Science Foundation, Grant HL-26890 from the National Institutes of Health, and Grant Li-256 from the Deutsche Forschungsgemeinschaft (DFG). Address for reprints: Murina J. Levesque, Ph.D., Physiological Fluid Mechanics Laboratory, Department of Mechanical Engineering, University of Houston, Houston, Texas 77004. Received November 5,1984; revision accepted November 12, 1985. 220 ENDOTHELIUM AND WALL SHEAR STRESS Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 vessels (i.e., away from branch points) the endothelial cells are elongated and aligned with the axis of the vessel, which is presumed to be the direction of blood flow. In regions of branching, however, the endothelial cell pattern not only is altered but also appears to be indicative of a time-averaged flow pattern in the immediate vicinity of the endothelium which would be consistent with that anticipated on the basis of fluid mechanic consideratons. Not only do the cells appear to align themselves with the flow direction, but the endothelial cells also appear to be more elongated in regions presumably characterized as high shear. Recently, in vitro studies of endothelial cellular dynamics have been initiated using cultured populations of vascular cells. Dewey et al. 12 have reported the use of a rotating cone-plate viscometer to study cultured bovine aortic endothelial cells under conditions of a uniform fluid shear stress. These studies suggest a shear stress effect on cell orientation, and more recent studies13 indicate that there are cytoskeletal changes and that the uptake of horseradish peroxidase by endothelial cells is dependent on a change in the level of shear stress. In our own laboratory we are using a parallel plate, channel flow device for similar studies. Our initial studies have also used cultured bovine aortic endothelial cells (fifth through tenth passage) on a plastic or glass substrate; those cells are exposed to a constant shear stress in the range of 8 to 85 dynes/cm2 for a time period of 1 to 24 hours. These results14 clearly indicate a shear stress effect on both orientation and cell shape, but one which is dependent on substrate attachment. Thus there is clear indication that hemodynamic wall shear stress does influence endothelial morphology and orientation. There is, furthermore, a suggestion of a direct correlation between the level of wall shear stress and the shape of the cell, with more elongated cells occurring in regions of higher wall shear stress. The present study was initiated to test this hypothesis further using an in vivo experimental model where the hemodynamics or fluid mechanics is altered in a predictable way through a change in vessel geometry. The model chosen for this study was that of dog aortic stenosis. The local endothelial geometry was then analyzed to determine whether or not the associated pattern of cell shape was consistent, at least qualitatively, with the fluid mechanic details of a stenosed flow. In addition, to examine the relationship between endothelial cell shape and fluid dynamics in a more quantitative way, laser Doppler anemometry studies of the velocity field were carried out in a model of a stenosis made from one of the vascular casts. The methods and the results are described in the next few sections. Before doing that, it should be noted that several investigators have studied the nature of flow in a stenosis. In vivo experimental investigations of arterial stenosis have largely been limited to measurements of hemodynamic parameters.15"19 However, there also have been a number of studies using flow through model geometries. Although largely limited to rigid models, these have allowed for the investigation of the more detailed fluid dynamic characteristics. These have included observations of the streamline pattern using dye, aluminum particles, or the hydrogen bubble technique,20"22 and measurements of pressure23"25 and velocity.22'25"29 There have also been numerical studies of flow through a stenosis. 3031 From all these studies, we know that in a weak stenosis there is very little influence on the velocity field; however, in a severe stenosis, there will be elevated shear stresses in the "throat," i.e., the most constricted part of the stenosis, Levesque et al. 221 and immediately distal a jet will form which separates from the wall only to reattach further downstream. The region between the separated jet and the wall will contain a recirculatbn zone in which the flow will be slowly moving in a vortical pattern and the level of wall shear stress will be on the low side. We also know from the above noted studies, however, that the exact details of flow through a stenosis (e.g., the coherent structure, turbulence, and the recirculation zone) are dependent on the Reynolds number, the Womersley parameter, a, and the degree and exact geometry of the stenosis. It was for this reason that we decided to conduct our own fluid dynamic studies in a model stenosis made from one of our vascular casts. Methods In Vivo Studies Ten mongrel dogs weighing 10 to 12 kg were used in this study. Six served as controls and four underwent surgical procedures to introduce an aortic stenosis. Animals were maintained in quarters approved by the American Association for Laboratory Animal Care, and the procedures followed were in accordance with institutional and National Heart, Lung, and Blood Institute guidelines. The animals were fully anesthetized with Nembutal (30 mg/kg body weight intravenously) and Xylazine (5 mg/kg intramuscularly). During the surgical procedure, isotonic Ringer was infused (2 ml/min), and respiration was maintained by a Harvard respirator (VT = 200 cc; 16 strokes/minute). The surgery was performed under sterile conditions in the surgical area in the animal care facilities specifically assigned for chronic experimentation. The thorax was opened at the level of the first set of intercostal vessels through a 5 cm long incision between the ribs. Through this opening, a cotton band (0.3 mm wide) was placed around the aorta. The band was tightened until the presence of a thrill or a bruit was felt distal to the band. This indicated that the stenosis created flow disturbances. In this way, the aortic cross-sectional area was decreased by 7 1 % ± 3%, and a small poststenotic dilation was also present. The animal was fully awake 4 to 6 hours after the procedure and was maintained free of pain with Xylazine (2 mg/kg intramuscularly) until the animal recovered its full level of activities. For 5 days, the animal received penstrep (0.05 ml/kg intramuscularly). The care for these animals was provided by the investigator and included outdoor walks twice daily (7 days/week, morning and evening). Upon returning from their walk, the animals were rewarded with a treat (8 oz of high protein meat product). Dry dog chow and water were provided at all times, and the animals were kept under sanitary conditions in the animal care housing facilities. The animals were sacrificed 12 weeks later with an overdose of Nembutal, and their aortas were prepared by the vascular casting procedure.8 From these vascular casts the cross-sectional area was calculated from diameters measured with a caliper in two planes, perpendicular to one another, at locations proximal, distal and in the stenosed portion. The average hydraulic diameter was used to calculate the cross-sectional area at selected sites. Micrographs of endothelial cells were obtained from the ventral aspect of the cast at various locations by using light microscopy (Olympus BH). Endothelial cells were analyzed with a Videoplan image analyzer (Cart Zeiss, Incorporated). The following parameters were obtained: area, perimeter, length, width, and the shape index (4narea/perimeter2). These parameters have been defined else- 222 ARTERIOSCLEROSIS VOL. 6, No 2, where.9 The analysis includes calculating these values for each cell in a sample population and then determining the statistical mean for that group of cells. In this report only the two most relevant parameters, area and shape index, are presented. Laser Doppler Studies Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 An elastic 1:1 true-to-scale silicon rubber model of a stenosis was constructed from one of the vascular casts of a dog aortic stenosis where the severity of the stenosis was 7 1 % based on area. The unstenosed diameter of the model was 10 mm and the wall thickness was 1 mm. This resulted in a modulus of elasticity of E = 1.1 x 10s dynes/cm2 and provided for a distensibility and wall motion that was a reasonable approximation of that of the dog aorta. The fluid used in the LDA studies was a glycol-water solution with a kinematic viscosity of T| = 8.5 centipoise. This viscosity is higher than that of blood; however, it was necessary to use this to achieve the same refractive index for the fluid as the wall of the silicon rubber model (n = 1.413). The entire model was embedded in the same fluid so that the path of the laser beam would not be deflected. The mean Reynolds number, however, was 450 and should be a reasonable representation of the flow through such a stenosis. Figure 1 illustrates the experimental set-up. The fluid was pumped from a receiver into a reservoir. To maintain a constant fluid pressure in the complete system during the measurements, any fluid surplus was piped back to the receiver via an overflow pipe. The mean volume flow rate was adjusted by positioning a small regulator tank. The entrance tube was 100 diameters in length, and the flow was thus fully developed. The pulsatile ftawwas generated by a piston pump. A continuous adjustable motor produced the sinusoidal waveform. A reservoir which acted as a "windkessel" was installed between the cylinder and the plexiglass tube. This served as a low pass filter suppressing the high frequency interference from the sine-wave mechanism, while passing the piston frequency. The velocity measurements were carried out using a laser Doppler anemometer. The principle of the laser Doppler anemometer has been described in detail by Durst et al. 32 and Uepsch.33"35 For these measurements a one-component laser Doppler anemometer with a 5 mW He-Ne laser was used to measure the local flow velocities in the axial direction. The measurements were carried out using the forward scattering method. The direction of the flow velocity was determined by producing a frequency shift between two beams by means of acousto-optic modulation using two Bragg cells. The LDA sample volume was approximately 10""3 mm3 and the axis of the ellipsoid resulting from the two beams was 50 /xm. Polystyrene balls of 0.5 /xm diameter were added to the fluid in a very low concentration. These are of nearly the same density as the fluid, are of high drag, and thus follow the fluid movement with virtually no slippage. The laser anemometer was mounted on an X-Y table. By shifting the anemometer perpendicular to the centeriine of the model, a complete velocity profile covering the entire diameter or lumen of the model vessel was obtained. The velocity profiles under steady flow conditions were plotted on-line to provide a continuous representation of the variation of velocity with position across the lumen of the model vessel. The spatial resolution of these measurements was ±50 Mm. Under the pulsatile flow conditions de- MARCH/APRIL 1986 scribed below, analog velocity output signals from a frequency tracker, which provided a voltage proportional to the frequency of the input signal, were recorded and digitized using an A/D converter and a process control computer. The average velocity and the RMS velocity fluctuations, over several periods (10 cycles) at one measurement point and at a constant phase angle, were obtained and recorded on magnetic tape. A time-mark light was attached to a plexiglass disk on the sine-wave mechanism. The position of this time mark could be adjusted at 22.5° intervals. The optoelectrical scanning device, consisting of a photoelectric barrier and a Schmitt trigger, generated a rectangular pulse whenever the angle mark was passed. The negative edge of this signal simultaneously triggered the A/D converter and the oscillographs, and the scanned signals were recorded on the plotter. In the pulsatile flow studies, the mean pressure was 100 mm Hg, the mean pressure gradient was 1480 Pa/m, and the superimposed sinusoidal oscillation had an amplitude of ± 20 mm Hg. For these studies, the frequency used was 1.23 Hz; the Womersley frequency parameter a = R (CD/V)1"* had a value of 6.5; the mean Reynolds number was approximately 450; and the maximum Reynolds number, 910. The radial positions of the measuring points were always selected so that there would be seven points across the tube diameter including one at the centeriine. For example, for a tube diameter of 10 mm (at the measuring point 30 mm proximal to the stenosis), the measuring points across the diameter were at distances of - 4 . 3 , - 3.3, - 1 . 6 , 0, 1.6, 3.3, 4.3 mm relative to the centeriine; while at a position with a tube diameter of 6 mm, the distances were - 2.6, - 2 , - 1 , 0 , 1 , 2 , and 2.6 mm. Flow studies were also carried out with a non-Newtonian fluid that was a mixture of AP 30 (0.05%) and AP 45 (0.04%) with MgCI2 (0.01%) and 4% isopropanol. The MgCI2 was added to provide a refractive index that would be nearly equal to that of the silicon rubber model, and both the MgCI2 and the isopropanol are agents which chemically stabilize the mixture. The complex viscosity was determined with a Haake rotating viscosimeter of the Couette type. This mixture has nearly the same viscosity as human blood with a hemocrit of 45%. The viscous component for this fluid corresponds to that for blood, whereas the viscoelastic component shows some differences. Although the viscosity of the Newtonian and non-Newtonian fluids was different, the Reynolds number was virtually the same (Re = 450). The velocity pattern results obtained were quite similar and quantitatively showed little difference. This is in contrast to previous studies by one of the authors34 and is believed to be due to the relative simplicity of a stenosis geometry as compared to the branch geometry used in earlier studies. Thus, the non-Newtonian fluid results provided little, if anything, different for the geometry used and are not discussed any further or used in later analyses here. From the velocity measurements for steady flow, the velocity gradients near the wall were determined and the shear stress, xw, was calculated. This was done by using the velocity measurement at the closest point, which was 100 fim from the wall for steady flow. The determination of shear stress from the model experiments then must be scaled to the in vivo conditions corresponding to the endothelial cell shape data. If Re hv)v0 = R e m ^ , then: (D ENDOTHELIUM AND WALL SHEAR STRESS Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 1. 2. 3. 4. Receiver Pump Reservoir Overflow Container 5. Valves 6. Model 7. Air Tank Levesque et al. 8. Regul. Tank 9. 5 mW He-Ne Laser 10. Photomultiplier 11. Bragg Cells 12. Sine Mechanism 13. Trigger 223 14. Pressure Tank 15. Reduction Valve 16. Compressor 17. Manometer Figure 1. Schematic diagram of experimental set-up used in laser Doppler anemometer studies of steady and pulsatile flow through a model stenosis. Equation (1) indicates that it is the ratio of shear stress to dynamic pressure, Vb pu2, that is constant in going from the model system to in vivo conditions. Since Re = pUD//i (where U represents the mean velocity and D is the diameter) and the diameter of the model is identical to that in vivo, then equation (1) can be rewritten as: ( \Mmode) • 2 /pmodel (2) \PinvivD where /t is the fluid viscosity and p is the fluid density. Although such a determination of the shear rate and the shear stress at the wall based on the velocity measurement at the closest point to the wall is only approximate, it does give a quantitative indication of the shear stress environment to which the endothelial cells were exposed. Results In Vivo Studies Endothelial cells from various locations along aortic stenoses with cross-sectional area reductions of 7 1 % ± 3% (range, 69 to 74) were analyzed and the results are shown qualitatively in Figure 2 (A to D) and quantitatively in Figures 3 and 4. In these, endothelial cells located far proximal to the stenosed area may be seen to have the same size and shape as the endothelial cells from unstenosed aortas. However, in more immediate proximity to the "throat* of the stenosis, the size and the shape of the cells have changed dramatically. In the convergent area of the stenosis, just proximal to the throat, the shape index decreased, and the cells were very elongated (Figure 3). The appearance of a cell (i.e., the degree of elongation or roundness) was reflected by the value of its shape index. By definition, a circle has a shape index of 1.0 and a straight line has a shape index of 0.0. Therefore, the smaller the shape index, the more elongated a cell is. In this region immediately proximal to the throat, the cells were also much smaller in area as is evident in Figures 2 B and 4. In contrast, in the divergent area, i.e., just distal to the throat of the stenosis, the cells were rounder with no specific orientation, and their boundaries had a higher affinity for silver straining (Figure 2 C). The values for the shape index increase suddenly and then subsequently decrease as seen in Figure 3. The largest cells were located 2 radii distal to the throat (Figure 4); however, at a more distal site (i.e., 8 radii) the cell morphology was not significantly different from control or prestenotic values. In the region where a thrill and a bruit were felt (approximately 5 radii distally), endothelial cell patterns resembling vortex-like 224 ARTERIOSCLEROSIS VOL 6, No 2, MARCH/APRIL 1986 Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 C D Figure 2. Light micrographs of endothelial cell patterns obtained from stenosed aortas at selected locations. A. 3 diameters proximal to stenosis. B. Throat of the stenosis. C. Immediately distal to stenosis. D. 2 diameters distal to stenosis. Arrow indicates direction of flow; Bar = 100 urn. ENDOTHELIUM AND WALL SHEAR STRESS structures were observed (Figure 2 D). In this area, a mixture of cells with different morphology (I.e., area and shape index) was found. These are responsible for the hump shown in Figure 3. Figure 5 presents the velocity profiles obtained for steady flow in the model stenosis where the Reynolds 0.8 r Stenosed Flow Experiments Control Experiments x T3 Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 a 225 number proximal to the stenosis in the region corresponding to the normal aorta of the dog is approximately 450. Proximal to the stenosis, we obtained the normal HagenPoiseuille profile. However, as the tube narrowed and the tube diameter decreased, the velocity increased, and the peak velocities in the stenosis region of the model were on the order of 150 cm/s. Near the vessel wall, high fluctuations in velocity were observed. The separation point, as determined by the onset of flow reversal at the wall, lay about 3 mm (0.3 diameter or 0.6 radii) distal to the throat, i.e., the point of the smallest diameter or the center of the stenosis. Because the stenosis and thus the cast were not exactly symmetric, there are velocity differences between the upper and lower wall as can be seen in Figure 5. The reverse flow zone initially grew, and its maximum thickness lay about 4 to 6 radii downstream or distal to the stenosis. The reattachment point, on the other hand, did not occur until approximately 6 diameters or 12 radii downstream. The growth of the separation region and the location of the reattachment point depended on the form of the Laser Doppler Anemometer Studies • O Levesque et al. 0.4 CO o o 0.2: 6- E ,U / a. 8 -o R 4- < < D P 2 Flow -0 8 -0 4 -0 2 1 02 0 X/D c \ — ) r ^ ^ 3 6 .4 8 X/D N 1 —-_^- i 8 D Figure 4. Mean cell surface area for controls and as a function of distance along the stenosis measured in units of radii proximal ( - ) and distal ( + ) . C = control; S = stenosis; P = proximal; D = distal; R = radius; and bar = std. Figure 3. Mean shape index of endothelial cells as a function of distance along the stenosis measured In units of radii proximal ( - ) and distal ( + ) . P = proximal; D = distal; R = radius; and bar = standard deviation. -3 1 1 Figure 5. Velocity profiles for steady flow of a Newtonian fluid through a stenosis made from a vascular cast and for selected positions both proximal and distal to the stenosis. X/D = distance proximal and distal to the stenosed area as a ratio of diameter of unstenosed vessel. 226 ARTERIOSCLEROSIS VOL 6, No 2, Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 stenosis and the Reynolds number, and thus cannot be compared directly with measurements found in the literature. However, the tendency is similar. The pulsatile flow results are illustrated in Figure 6 for four different locations, two on the proximal side ( - 30 and - 4 mm upstream of the throat of the stenosis) and two on the distal side (4 and 6 mm downstream of the throat). As may be seen, on the upstream side at the most proximal position, the profile, though pulsatile, at any instant of time approximated that of Poiseuille flow. As the flow entered the stenosis, the velocity increased and there was no flow separation from the wall in this proximal region at any time during the entire cycle. Distal to the stenosis at locations of 4 and 6 mm downstream, reverse flows were found throughout the entire cycle as may be seen in Figure 6. At these locations, flow separation was also found in the steady flow studies. In general, with the obvious exception being the time-varying magnitude of the velocities, the flow patterns as measured for steady flow and pulsatile flow were qualitatively the same. As was mentioned earlier, this was also true of results obtained with a non-Newtonian fluid. Furthermore, cell culture studies indicated that the time needed to orient the direction of a cell is on the order of hours, not fractions of a second. This, coupled with the similarity in results between steady vs pulsatile flow studies and between Newtonian vs non-Newtonian fluid studies, has led us to only use the Newtonian steady flow results for further analysis. It should be noted, however, that, in a more complicated geometry, non-Newtonian effects may be important. Also, although flow pulsatility is not believed to be an important determinant of cell shape and orientation, transient fluid dynamic phenomena may have an important influence on cell function.13 R» - 4 8 0 , a - 8 . S , f - 1.23 Hz N«wtoni*n Fluid 270°- 225' 1B0° 135' Figure 6. Velocity profiles as a function of position in cycle for pulsatile flow of a Newtonian fluid through model stenosis and for selected positions both proximal and distal to stenosis; upper left shows the time-dependence of pressure gradient. Re = Reynolds number; a = Wormersley parameter; and f = frequency. MARCH/APRIL 1986 Discussion Our technique has enabled us to produce a short axisymmetric stenosis of moderate severity with a 71 % crosssectional area reduction in the dog upper thoracic aorta. The stenosis was created 12 weeks prior to sacrifice to ensure that any injury and physical trauma caused by the surgical procedure would be repaired. In fact, we believe that the endothelium was traumatized by the surgery and that the measurements made 12 weeks later were on a new, regenerated endothelium. The animals remained healthy and active during the course of the experiment. At the time of sacrifice, endothelial integrity was maintained all along the stenosed aortas. The vascular casting technique was chosen mostly because of its small amount of shrinkage as well as the maintenance of the three-dimensional geometry of the specimen. It is preferable to other traditional methods of studying the endothelium when quantitative information is required. 69 From our previous study, we have shown that morphometric changes occur near branching vessels.11 Therefore, to avoid morphometric bias that would be introduced by the presence of intercostal branches, the analysis was limited to the ventral aspect of the vascular casts. The presence of a stenosis in an artery alters the flow field in the artery both locally and distally. Flow instability and turbulence can occur in the neighborhood of arterial stenosis, giving rise to a vascular murmur. In general, the blood velocity in the stenosed area increases and the pressure decreases.19'M Distal to the stenosis, the flow separates from the wall and a negative velocity is seen as part of the annular recirculation zone. Further downstream, the flow recovers and eventually becomes like a Poiseuille flow. This representation is based on the assumption that the flow is laminar and steady. The pulsatile nature of the arterial flow during a cardiac cycle will change this picture. For example, it should be noted that for pulsatile flow, flow reversal during the cycle will occur and the location of flow separation and reattachment points will move toward and backward. Nevertheless, the results described in the last section suggest little qualitative difference between the basic character of steady versus pulsatile flow through a stenosis. Also, cell culture studies suggest that the orientation of a cell is not responsive to transient hemodynamic conditions with a time-scale characteristic of a normal cardiac cycle. We thus believe that, if the endothelial cell pattern is to be indicative of the flow pattern in the immediate vicinity of the arterial wall, it then is the mean flow suitably averaged over some length of time that is reflected. Therefore, our results are discussed here in the light of hemodynamic conditions expected for steady flow through a rigid stenosis. Furthermore, based on the similarity between studies with a Newtonian vs a non-Newtonian fluid, as noted previously, it is the results for the steady flow of a Newtonian fluid through a model stenosis that are used for further analysis. The results from this study show that endothelial cells are smaller and more elongated (i.e., have a smaller shape index) in the throat of a stenosis. It is the throat of a stenosis where the velocity would be high and the wall shear stress the highest. In the region immediately distal to the stenosis, the endothelial cells are larger and have a more rounded appearance which is reflected by a larger value for their shape index. Two radii downstream from the stenosis point (i.e., the throat) the endothelial cell shape index values are close to control values, although their surface area is still greater than that for control cells. It is believed ENDOTHELIUM AND WALL SHEAR STRESS Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 that the region located distal to the stenosis point corresponds to the recirculation zone where reversed flow is likely to occur. Further downstream (4 to 6 radii) in the region where a thrill and a bruit were felt, endothelial cell patterns resembling vortex-like structures were observed (Figure 2D). These patterns have centers made up of rounder cells with elongated cells surrounding them. We believe that these patterns reflect unusual flow patterns which could include the possibility of flow recirculation and severe fluctuations. The rounder cells have a larger surface area and their boundaries have a higher affinity for silver staining. In comparison with other studies, rounder endothelial cells are found in regions suspected of low shear stress.11'14' 38~37 It is possible that rounder cells may indicate some form of cellular damage in spite of the fact that en face endothelial integrity is maintained. These cells may have a higher level of metabolism and permeability. Furthermore, rounder cells (regardless of their location) are multinucleated and occur in areas of repeated injury or high turnover.38^*3 The most striking feature of the results shown in Figure 3 are the extremely elongated cells in the "throat" of the stenosis, followed almost immediately downstream by very round cells. In a region of just a few millimeters, the shape index goes from a value of 0.1 to 0.74. In all the studies that we have conducted using the vascular casting technique to determine arterial endothelial cell shape, we have never seen as low or as high a value as represented by these two extremes. Yet these results are entirely consistent with the fluid mechanics in the context of the hypothesis. In the throat the wall shear stress should be the highest; however, downstream, when the jet separates, there should be a very low level of shear stress associated with the recirculation zone. The relationship between endothelial cell elongation and wall shear stress can be examined further by comparing the data in Figure 3 with wall shear stress values calculated from the steady flow velocity profiles in Figure 5. The latter is shown in Figure 7 where the wall shear stress is presented as a function of X/D through the stenosis. The wall shear stress is nondimensionalized by a value, T0, which is calculated using a Poiseuille parabolic profile with a Reynolds number of 450 and the unstenosed diameter of 10 mm. Using the density and viscosity for the model fluid, T0 is calculated to be 26 dynes/cm2. The ratio zjxo is pre- i LOWIR WALL ! O UPPtR WALL - 227 Levesque et al. sented for both the upper and lower wall. As may be seen, there are some differences between these two locations due to the fact that the stenosis is not exactly symmetric; however, qualitatively they are similar and quantitatively the shear stress values are close. These values tend to confirm that it is at the throat where the wall shear stresses are the highest. In the proximal region, over a distance of 4 radii or two diameters in length, there is a gradual increase in the nondimensional wall shear stress to a peak value of almost 10 at the throat. However, downstream of the throat the shear stresses literally plummet to values of T.JT0 again on the order of one. This variation in wall shear stress shown in Figure 7 is the inverse of that shown for the cell shape index in Figure 3, and further suggests that cells are more elongated in regions of high shear stress and more nearly round in regions of low shear stress. To show this relationship between cell shape and wall shear stress further, Figure 8 presents data on the variation of the inverse of the shape index with the wall shear stress, TW. These data are obtained through a combination of results from Figures 3 and 7. However, only a few of the points represent cases where both the cell shape and velocity profile (and thus shear stress) were measured at the same X/D. Thus, when for a given measurement of inverse shape index there was no corresponding velocity profile measured from which wall shear stress could be calculated, an interpolation of the data in Figure 7 was used to determine t w . Similarly, where no cell shape data were available at a location where we had a velocity profile and thus a wall shear stress, an interpolation of the data in Figure 3 was used to determine the inverse shape index. Although this perhaps may be questioned, the results in Figure 8 suggest that there is a strong correlation between the elongation of an endothelial cell and the local wall shear stress. This is certainly true in the range of shear stress between 5 and 50 dynes/cm2. Below 5 dynes/cm2, where all of the data are from the recirculation zone, the data are too scattered to support any conclusion, particularly considering the interpolation necessary to produce this figure. There is one cautionary note. The wall shear stress values have been obtained in a model stenosis where the mean Reynolds number in the unstenosed portion of the aorta is 450. Although such a value for the Reynolds number is representative of the dog aorta, it is an assumed •HEAR >TREM 3 X/D Figure 7. Variation of mean wall shear stress with position through model stenosis based on calculations using steady flow, Newtonian fluid velocity profiles from Figure 5. X/D = distance proximal and distal to the stenosed area ratioed to the diameter of the unstenosed vessel. 15 20 25 30 35 45 2 Wall Shear Stress,T~dynea/cm Figure 8. Con-elation of measured inverse shape index of endothelial cells with calculated mean wall shear stress based on combining Figures 3 and 7. 228 ARTERIOSCLEROSIS V O L 6, No 2, value. If in a given animal, the flow rate and thus the Reynolds number were higher, then the shear stress would also be higher. In this case, the data points in Figure 8 would have to be shifted to the left. Another assumption used in the conduct of the model stenosis flow study was that far upstream the flow was fully developed with the classical Poiseuille parabolic velocity profile. If the actual in vivo profile were blunter, this would probably have little effect on the flow in the region of the throat or further downstream. However, upstream or proximal to the throat, there could be an effect and shear stress values would be higher. This would tend to steepen the appearance of the proximal to stenosis data in Figure 8, i.e., lower shear stress points would be moved more to the right than the higher shear stress points. Qualitatively, however, the trend would be the same. Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 It should also be pointed out that there is a variation in circumferential wall tension and strain along the stenosis. LaPlace's law for a cylinder states that the wall tension (force/unit length) is equal to the product of pressure multiplied by vessel radius. This indicates that there would be a smaller wall strain in the stenosed than in the unstenosed region of the aorta. In fact, the banding of the aorta produces a rather rigid stenosis region, whereas away from the stenosis the aorta is free to distend and will have normal values for circumferential strain. Although one cannot preclude this exerting some effect on the geometry of the vascular endothelial cells, there are no data available and this has not been taken into account in the analysis of the present data. In spite of these limitations, the study shows that aortic endothelial cell morphology can be altered in vivo by a vascular geometric change that is known to influence local hemodynamics. It may be seen that the shape index distribution along these stenosed vessels can be correlated to the level of wall shear stress theoretically expected, i.e., the endothelial cells are more elongated in regions of high shear and more rounded in regions of low shear. There is substantial evidence based on other studies that shows that high flow rates (high shear stresses) tend to protect against atherosclerotic lesion formation,44 and that atherosclerotic lesions tend to be accentuated in regions of low flow velocity.45 Furthermore, there is also a suggestion that lesions preferentially occur in regions of more rounded endothelial cells38 and also that cell turnover is enhanced in areas of rounder endothelial cells.40 It is in this context that we believe that vascular geometry — through its influence on hemodynamics, including wall shear stress, and on endothelial cell shape — may be a major factor in the determination of the pattern of atherogenesis. References 1. Mitchell JRA, Schwartz CJ. Arterial disease, Oxford, England: Blackwell Scientific Publishing Ltd, 1965 2. Montenegro MR, Eggen DA. Topography of atherosclerosis in the coronary arteries. Lab Invest 1968;18:586-593 3. Caro CG, Fltz-Gerald JM, Schroter RC. Atheroma and arterial wall shear: Observation, correlation and proposal of shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond (Biol), 1971 ;117:109-159 4. Nerem RM, Levesque MJ. The case for fluid dynamics as a localizing factor for atherogenesis. In: Schettler G, Nerem RM, Schmid-Schonbein H, Mdri H, Diehm C, eds. Fluid dynamics as a localizing factor for atherosclerosis. Berlin: Springer-Verlag 1983:26-34 MARCH/APRIL 1986 5. Friedman MH, Deters OJ, Mark FF, Bargeron CB, HutchIns QM. Arterial geometry affects hemodynamics — A potential risk factor for atherosclerosis. Atherosclerosis 1983; 46:225-231 6. Flaherty JT, Pierce JE, Ferraris VJ, Patel DJ, Tucker WK, Fry D L Endothelial nuclear patterns In the canine arterial tree with particular reference to hemodynamic events. Circ Res 1972;30:23-33 7. Sllkworth JB, Stehbens WE. The shape of endothelial cells in en face preparations of rabbit blood vessels. Angiology 1975;26:474-^87 8. Levesque MJ, Comhlll JF, Nerem RM. Vascular casting: A new method for the study of the arterial endothelium. Atherosclerosis 1979:34:457-^67 9. Cornhlll JF, Levesque MJ, Herderlck EE, et al. Quantitative study of the rabbit aortic endothelium using vascular casts. Atherosclerosis 1980:35:321-327 10. Rekfy MA, Levesque MJ. A scanning electron microscopic study of arterial endothelial cells using vascular casts. Atherosclerosis 1977:28:463-^70 11. Nerem RM, Levesque MJ, Cornhlll JF. Vascular endothelial morphology as an indicator of blood flow. ASME J Biomech Eng 1981:106:172-176 12. Dewey CF Jr, Bussolari SR, Glmbrone MA Jr, Davles PF. The dynamic response of vascular endothelial cells to fluid shear stress. ASME J Biomech Eng 1981:103:177-185 13. Dewey CF Jr. Effects of fluid flow on living vascular cells. ASME J Biomech Eng 1984;106:31-35 14. Nerem RM, Levesque MJ, Sato M. Vascular dynamics and the endothelium. In: Schmid-Schonbein GW, ed. Frontiers in btomechanics, New York: Springer-Veriag (in press) 15. Evans DH, Macpherson DS, Asher MJ, Bently S, Belln RRF. Changes in Doppler ultrasound sonograms at varying distances from stenoses. Cardiovasc Res 1982;16:631-636 16. Feldman RL, Nlchole WW, Edgerton JL, Corrtl CR, Peplne CJ. Influence of aortic stenosis on the hemodynamic importance of coronary artery narrowing in dogs without left ventricular hypertrophy. Am J Cardiol 1983:51:865-871 17. Sun Y, Most AS, Ohley W, Gewlrtz H. Estimation of instantaneous blood flow through a rigid coronary artery stenosis in anesthetized domestic swine. Cardiovasc Res 1982;17: 449-504 1B. Schwartz JS. Compliant coronary stenoses. Int J Cardiol 1983:4:315-318 19. Wong AYK, Klasses GA, Johnston* DE. Hemodynamics of coronary artery stenosis. Can J Physiol Pharmacol 1984;62: 59-69 20. Goldsmith HL, Karino T. Microrheology and chemical medicine: Unravelling some problems related to thrombosis. Clin Hemomeology, 1982:143-155 21. Matsuzawa T, Takayoshl F, Takehlko A. Visualization of flow in normal and morbid blood vessel models. Proceedings of the 3rd International Symposium on Flow Visualization, Ann Arbor, Sept. 6-9, 1983 22. Ahmed Saad A, Glddnes DP. Pulsatile poststenotic flow studies with laser Doppler anenomety. J Biomech 1984;17: 695-705 23. Clark C. Turbulent velocity measurements in a model of aortic stenosis. J Biomech 1976:9:633-640 and 1977;10: 4 6 1 472 24. Fredberg JJ. Origin and character of vascular murmurs: Model studies. J Acoust Soc Am 1977:61:1077-1085 25. Young DF, Tsal FY. Flow characteristics in models of arterial stenosis: steady flow and unsteady flow. J Biomech 1973; 6:395-410, 547-559 26. Mates ER, Gupta RL, Bell AC, Klocke FJ. Fluid dynamics of coronary artery stenosis. Circ Res 1978;42:152-162 27. Tomm D, Zeller H. The sound caused by pulsatile flow through arterial stenosis. International Colloquium Strouhal number Centenary. Libice (CSSR) June 27-30, 1978 28. Despande MD, Giddens DP, Mabon RF. Steady laminar flow through a modelled vascular stenosis. J Biomech 1976; 9:165-173 29. Zlmmer R. Geschwindigkeitsmessungen mit einem Laser- ENDOTHELIUM AND WALL SHEAR STRESS 30. 31. 32. 33. 34. Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 35. 36. Doppler-Anemometer in Modellen einer Mehschlichen Beinarterien ver Zweigung (Arteria Femoralis) bei Stationarer und Pulsierender Stromung. [Disseration] Technische Hochschule Munchen, 1982 Vlachos NS, Whitelaw JH. Low Reynolds number flow in the vicinity of axisymmetric constrictions. J Mech Engr Sci 1979; 21:73-84 Wille S, Walloe L. Pulsatile pressure and flow in arterial stenoses simulated in a mathematical model. J Biomed Engr 1983;31:17-24 Durst F, Melling A, Whitelaw JH. Principles and practice of laser-Doppler anemometry. London: Academic Press, 1976 Liepsch D. Flow conditions at 90° branchings as determined in glass models of small arteries by means of a laser-Doppler velocimeter. Proceedings of the 2nd Annual New England Bioengineering Conference. Worchester, Massachusetts, March 29-30 (1974) 486-499; and VDI Berichte 232 (1975); 423-^41 Liepsch D, Moravec S, Zimmer R. Pulsating flow in distensible models of vascular branches. In: Schettler G et al., eds. Fluid dynamics as a localizing factor for atherosclerosis. Berlin: Springer 1983,46-50 Liepsch D. Beruhrungsloses messen von stromungsgeschwindigkeiten mittels laser-Doppler-anenometer. HLH 1978; 341-344, 383-387 Nerem RM, Levesque MJ. Hemodynamics as a factor in lesion localization. In: Gotto AM, ed. Proceedings of the VI International Symposium on Atherosclerosis. Berlin: Springer-Verlag, 1983:361-369 Index Terms: atherosclerosis • endothelium Levesque et al. 229 37. Levesque MJ, Nerem RM. Vascular endothelial cell morphology in various species and its implications for arterial fluid dynamics. In: Hall CW, ed. Biomedical Engineering II — Recent Developments. New York: Pergamon Press, 1983: 110-113 38. Repin YS, Dolgov VV, Zaikina OE, Novikov ID. Heterogeneity of endothelium in human aorta — A quantitative analysis by scanning electron microscopy. Atherosclerosis 1984; 50:35-52 39. Bondjers G, Brattsand R, et al. Endothelial integrity and atherogenesis in rabbits with moderate hypercholesterolemia. Artery 1977;3:395-408 40. Caplan BR, Schwartz CJ. Increased endothelial cell turnover in areas of in vivo Evans blue uptake in the pig. Atherosclerosis 1973;17:401-417 41. Hansson GK, Bondjers G. Endothelial proliferation and atherogenesis in rabbits with moderate hypercholesterolemia. Artery 1980;7:316-329 42. Poole JCF, Sanders RG, Florey HW. The regeneration of aortic endothelium. J Pathol Bacteriol 1958;75:133-143 43. Stehbens WE. Endothelial cell mitosis and permeability. J Exp Physiol 1965;50:9O-92 44. Zarins CK, Bomberger RA, Glagov S. Local effects of stenosis: Increase flow velocity inhibits atherogenesis. Circulation 64(Suppl ll):1980;221-227 45. Zarins CK, Giddens DP, Bharedraj BK, et al. Carotid bifurcation atherosclerosis: Quantitative correlations of plague localization with flow velocity profiles and wall stress. Circ Res 1983:53:502-514 • hemodynamics • wall shear stress Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. M J Levesque, D Liepsch, S Moravec and R M Nerem Downloaded from http://atvb.ahajournals.org/ by guest on August 3, 2017 Arterioscler Thromb Vasc Biol. 1986;6:220-229 doi: 10.1161/01.ATV.6.2.220 Arteriosclerosis, Thrombosis, and Vascular Biology is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 1986 American Heart Association, Inc. All rights reserved. Print ISSN: 1079-5642. Online ISSN: 1524-4636 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://atvb.ahajournals.org/content/6/2/220 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Arteriosclerosis, Thrombosis, and Vascular Biology can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answerdocument. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Arteriosclerosis, Thrombosis, and Vascular Biology is online at: http://atvb.ahajournals.org//subscriptions/