* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Intracellular calcium concentration and calcium transport in
Stimulus (physiology) wikipedia , lookup
Multielectrode array wikipedia , lookup
Long-term depression wikipedia , lookup
Membrane potential wikipedia , lookup
Resting potential wikipedia , lookup
End-plate potential wikipedia , lookup
Single-unit recording wikipedia , lookup
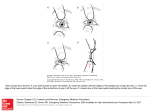
1032 INVESTIGATIVE OPHTHALMOLOGY 6 VISUAL SCIENCE / July 1985 Vol. 26 Introcellulor Colcium Concentration ond Colcium Transport in the Rabbit Lens Kenneth R. Highrower,* George Duncan, f and Sharon E. Harrison* Calcium-sensitive microelectrodes have been employed to determine that the free, intracellular concentration of calcium in the lens is approximately 30 nM. Additionally, active extrusion of intracellular calcium has been demonstrated. Invest Ophthalmol Vis Sci 26:1032-1034, 1985 Calcium ions appear to play several primary roles in lens physiology. An increase in lens calcium is accompanied by an increase in light scatter,1 a decrease in protein synthesis,2 and a destabilization of the lens cytoplasmic gel.3 The control of calcium movements by active and passive mechanisms are, however, difficult to investigate by conventional radiotracer methods partly because a large fraction of the total calcium appears to be bound 4 and partly because of the extremely slow penetration of 45Ca into the lens.5 In the present study, we employ calcium-sensitive microelectrodes to determine the distribution of free and bound calcium in the rabbit lens but, more importantly, we develop techniques to investigate active calcium extrusion from the lens. Materials and Methods. Rabbit lenses (4-5 wk) +60 + 80 00 8 7 6 5 4 3 pCa Fig. 1. Calibration curve for Ca++-sensitive microelectrode plots the pCa values as a function of the potential difference (mV) between the intracellular voltage and calcium-sensitive electrode (filled circles). A typical value from one lens measurement (x) is +45 mV, corresponding to a pCa of 4.5 (pCa = -logio [Ca++]) or 32 fiM. containing elevated levels of calcium are obtained by culturing lenses at 22°C in a HEPES-buffered medium (pH = 7.4) containing 5 raM glucose, 135-150 mM NaCl, 8 mM KC1, 3 mM MgCl2, and 20 mM CaCl 2 . Temperature reduction has been shown to facilitate a net accumulation of calcium without introducing poisons or metabolic inhibitors.1 Moreover, changes in the concentration of Na + and K + are minimized, particularly as compared with temperature reductions to 4°C. Osmolarity is adjusted to 300 ± 1 mOsm by varying the NaCl content. Calcium extrusion experiments are performed in TCI99 at 37°C. Calcium-sensitive microelectrodes are made according to methodology employed by Jacob, 4 and suitable electrodes are in the resistance range of 80200 megohms. All electrodes, voltage and ion-sensitive, are calibrated before and after lens measurements using a series of pCa buffers according to the method of Maraben et al,6 who introduced the terminology pCa to describe logarithmically the molar Ca ++ concentration. Thus the pCa3 buffer consists of 1 mM CaCl 2, no ligand, 98 mM KC1, and 10 mM MOPS (3-[N-morpholino]propanesulfonic acid, Sigma M1254) at pH 7.3. The pCa buffers 4, 5, 6, 7, and 8 all contain 5 mM CaCl2, 90 mM KC1, 10 mM ligand (NTA, NTA, HEDTA, EGTA, and EGTA, respectively) and 10 mM buffer—HEPES, pH 7.39; Tris, pH 8.42; HEPES, pH 7.7; HEPES, pH 7.29; and HEPES pH 7.8; respectively. The pCaoo solution consists of 10 mM EGTA, 100 mM KC1, and 10 mM HEPES at pH 7.6. Following pH adjustment with 1 N KOH, each buffer is passed through a 0.22ixm filter and stored in plastic at 4°C when not in use. In a typical experiment, immediately following electrode calibration, the lens is impaled with the reference microelectrode and the potential is monitored on channel A relative to an external reference electrode in the media. Within 10 min of a stable lens voltage, the calcium electrode is hydraulically manipulated into place and this potential is simultaneously monitored on channel B. We can also monitor the difference in potential (A - B) of the two electrodes as the calcium potential. Figure 1 shows a standard calibration curve (solid circles). The result of a typical lens measurement is also illustrated (X) in which the value of +45 mV is obtained from the differential amplifier as the difference between the reference Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933123/ on 08/03/2017 1033 Reports No. 7 Table 1. Intracellular Ca ++ in rabbit lens: influence of age and voltage* Lens condition Culture time (hr) [Ca++]r Young (4 wk) Mature (3 yr) Traumatized^ Ouabain£ 0 0 0 6 (nMtf [CV + ] F (nMtf [Ca++]r [Ca++]F [Na+]T (mM)\ 250 ± 50 499 ± 50 250 ± 50 187 ± 18 33.3 ± 2.5 51.3 ±6.8 34.0 ± 3.0 29.9 ±4.1 7.58 9.73 7.35 6.25 13.0 ±0.05 21.8 ± 3.45 13.0 ±0.05 42.1 ± 1.86 potential of - 6 0 mV and the calcium-electrode potential of -105 mV. This is equivalent to pCa 4.5 (—log10 [Ca ++ ] = 4.5) and corresponds to a calcium concentration of 32 /*M. The concentration of total calcium and other ions in the lens is measured by atomic absorption spectrophotometry (Perkin-Elmer, Model 272; Norwalk, CT) as detailed elsewhere.7 The use of animals in the present study conforms to the ARVO Resolution on the Use of Animals in Research. Results and Discussion. The free calcium concentration in the young rabbit lens of 33 /uM (Table 1) obtained in the present study represents 13% of the total and is significantly higher than 1 or 5 juM obtained in rat and frog lenses, respectively.3'4 This concentration is also approximately 100-fold greater than that found in nerve and muscle and might be expected to induce cytotoxic effects. For example, 10 nM levels close gap junctions in salivary gland epithelia.8 It should be remembered, however, that measurements from the lens were made in fiber cells, not metabolically active epithelial cells which contain an abundant supply of Ca-ATPase and are presumably capable of extruding more calcium. In view of the fact that this represents an unexpectedly high value for free calcium in the rabbit lens, we carried out calcium measurements under different conditions. Also, since the measurement of calcium-sensitive voltages depends on the magnitude of the membrane potential, we determined whether free calcium measurements were influenced by Recovery condition^ 0 time 6 hr 20 hr -64.8 -64.0 -53.0 -50.0 ± 0.84 ±0.71 ± 3.00 ± 1.70 % Vitreous removed. £ Cultured in 0.1 mM ouabain/TC199 at 37°C. * Values are expressed as means ± 1 SE for n = 4-8 lenses. t T signifies total; F signifies free, intracellular. Table 2. Recovery of Ca Voltage (m V) changes in the potential. In addition, we measured the voltages as a function of electrode depth. Table 1 shows that the free calcium measured in control and traumatized lenses were identical despite a 12 mV depolarization of membrane potential. In fact, a 25 mV depolarization induced by ouabain did not lead to a significant difference in the measurement. It is noteworthy that in the latter case the internal sodium concentration had increased threefold following ouabain poisoning, but the total calcium had not changed significantly. When an electrode first penetrates the lens, whether it is an intracellular or ion-sensitive electrode, the reading is not stable until an electrode seal is obtained. In the rabbit lens, steady values were obtained for both electrodes after a depth of approximately 20 /u.m was reached. In the present series of experiments, the free calcium values were stable for more than 10 min and did not in fact change significantly between depths of 25 and 250 /um. This indicates that the measurements were not contaminated by an extracellular leak component or influenced by tip blockage. For convenience, an electrode depth of 100 /urn was chosen for experimental purposes since very stable values were obtained at this depth while the electrode tended to block or break if depths greater than 300 /zm were attempted. Previously, Hightower and Harrison9 have shown that the total calcium content of the rabbit lens increased progressively with age. Subsequent studies on lens homogenates indicated that calcium binding sites may have increased with age. The present study -loaded lens* [Ca++]T [Ca++ h (vM). [Ca++]T [Ca++]F (mM)% Voltagi?(mV) 232.5 ± 54 83.7 ± 16 54.0 ± 7.0 6.5 8.5 10.2 13.0 ±0.22 14.7 ±0.56 17.6 ± 1.06 -51.8 ± 1.4 -57.0 ±2.3 -55.0 ±2.0 t 1,505 ±0.07 714 ±0.06 555 ± 0.04 * Values are expressed as means ± 1 SE for n = 4-8 lenses. t All lenses precultured for 24 hr in 20 mM Ca ++ , at 22°C; recovery in TCI99 at 37°C. % T signifies total; F signifies free, intracellular. Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933123/ on 08/03/2017 1034 INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE / J u l y 1985 b^ a — |'.I!:::::: I :::::£:t'iiiii! ' iiiii I .-ni z ----- -S.lllllliS :::::::::: ,1 =-. fffffffi irifi | ::::::* - • - . — d ~ 'Illir .tttti 1 :S:::::: II tty^^iiiiiiiiitiiy - Fig. 2. Photographs of young rabbit lenses during incubation for various periods of time in HEPES buffered media at 22°C containing 20 mM Ca ++ : a, zero time; b, 6 hr; c, 24 hr; d, 24 hr followed by 20 hr recovery in TC199 at 37°C. shows in Table 1 that both free and total calcium levels nearly double over a 3-year span, while the ratio of total/free increases by a factor of 1.3. This may suggest that redistribution of calcium occurs in the mature lens. It is interesting that this increase in internal calcium is not accompanied by a change in membrane potential as is typically characteristic of a change in membrane permeability. The level of free calcium in the lens represents a balance between the movement of Ca 2+ across the surface membranes and the exchange between internal binding sites. Calcium movements across the lens membranes have hitherto been studied exclusively using 4SCa radioisotope methods, and recently, Paterson and Delamere5 have shown that such studies are extremely difficult to interpret in view of the slow diffusion of calcium into the lens. To circumvent the shortcomings of tracer techniques in the study of active transport, we have used the ion-sensitive calcium electrode to monitor the movement of intracellular free calcium from the lens against an electrochemical gradient. Lenses were first precultured in a calcium-enriched, Hepes-buffered medium for 20 hr. During this time, the total calcium in the lens increased from 250 to 1,505 fxM, while the free calcium increased from 33 to 232 yM (Table 2). During this incubation, there Vol. 26 was little change in sodium content and only a 10 mV depolarization in membrane potential. There was no significant hydration of the lens as in previous experiments, but the lens cortex was opaque at the end of the incubation period (Fig. 2). During subsequent culture in TCI99 at 37 ° Q the voltage hyperpolarized to within 85% of the control value (Table 2), and lens transparency was restored. More importantly, there occurred a decrease in free calcium from 232 to 84 ^M within 6 hr and a further decline to 54 uM at 20 hr. Intracellular calcium was therefore extruded from the lens against a significant electrochemical gradient, indeed, under conditions where the electrochemical gradient was increasing. Since the external calcium concentration is 2,000 ;uM and the membrane potential was nearly —60 mV calcium extrusion occurred against a thermodynamically equivalent concentration ratio of 9,000. This is unequivocal evidence that active calcium extrusion takes place in the rabbit lens. Key words: intracellular calcium, transport, microelectrode, lens, opacification From the Institute of Biological Sciences,* Oakland University, Rochester, Michigan; and the School of Biological Sciences,! University of East Anglia, Norwich, United Kingdom. Supported by National Eye Institute research grant EY-03680. Submitted for publication: October 19, 1984. Reprint requests: Kenneth R. Hightower, PhD, Institute of Biological Sciences, Oakland University, Rochester, Ml 48063. References 1. Hightower KR and Dering M: Development and reversal of lens opacification caused by calcium. Invest Ophthalmol Vis Sci 25:1108, 1984. 2. Hightower KR: The influence of calcium on protein synthesis in the rabbit lens. Invest Ophthalmol Vis Sci 24:1422, 1983. 3. Duncan G and Jacob TJC: Calcium and the physiology of cataract. 1984 Human Cataract Formation. Pitman, London, Ciba Foundation Symposium 106, in press. 4. Jacob TJC: A direct measurement of intracellular free calcium within the iens. Exp Eye Res 36:451, 1983. 5. Paterson CA and Delamere NA: An analysis of 45Ca fluxes in the rabbit lens. Curr Eye Res 2:727, 1982/1983. 6. Maraben E, Rink TJ, Tsien RW, and Tsien RY: Free calcium in heart muscle at rest and during contraction measured with Ca++-sensitive microelectrodes. Nature 286:845, 1980. 7. Hightower KR, Leverenz V, and Reddy VN: Calcium transport in the lens. Invest Ophthalmol Vis Sci 19:1059, 1980. 8. Lowenstein WR and Rose B: Calcium in (junctional) intercellular communication and a thought on its behavior in intracellular communication. Ann NY Acad Sci 307:285, 1978. 9. Hightower KR and Harrison SE: Changes in the Ca ++ , Mg ++ and SH content in the developing rabbit lens. Lens Res 1:249, 1983. Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933123/ on 08/03/2017