* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Treatment of Swine Flu
Survey
Document related concepts
Eradication of infectious diseases wikipedia , lookup
Oesophagostomum wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Ebola virus disease wikipedia , lookup
Leptospirosis wikipedia , lookup
Hepatitis C wikipedia , lookup
Trichinosis wikipedia , lookup
West Nile fever wikipedia , lookup
Marburg virus disease wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Hepatitis B wikipedia , lookup
Orthohantavirus wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Henipavirus wikipedia , lookup
Oseltamivir wikipedia , lookup
Transcript
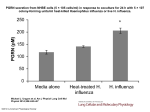
Swine Flu ( H1N1"2009" Virus ) Background Influenza is a respiratory illness caused by orthomyxoviruses. The word influenza may have been derived from the Latin word influo, which means "to flow in," indicating airborne transmission, or from the Italian word influence, which indicates influence of weather. Influenza viruses are RNA enveloped viruses ,that have eight pieces of segmented RNA genome, and consist of Influenza A,B, and C viruses . Influenza type A viruses cause disease in humans and animals; birds are the natural reservoir for type A ,and may cause worldwide epidemics (Pandemics). Influenza type B viruses primarily cause disease in humans, particularly children. Infections with influenza type C viruses are rare. Important Properties: Influenza virus is composed of: 1-A segmented single strand RNA genome 2-Helical neucleocapsid 3-Outer lipoprotein envelope which is covered by two different spikes (Hemagglutinin and Neuraminidase). Influenza viruses have both group –specific and type –specific antigens : 1-The internal ribonucleoprotein is the group –specific Ag (Influenza A, B and C ) 2-The HA and NA are type – specific Ags located on the surface . Many species of animal (aquatic birds ,swine ,chicken ,horses …..) have their own influenza A viruses. Antigenic Changes: Influenza viruses especialy influenza A show changes in antigenicity of their HA and NA proteins ,this property contributes to their pandemics .There are two types of antigenic changes: 1-Antigenic shifts where reassortment of segments of genome RNA(entire segment of RNA exchanged ). 2-Antigenic drift where minor changes due to mutations in RNA genome. The animal viruses are the sources of the RNA segments that encoded the antigenic shift variants that cause epidemics among human , for example if avian and human influenza A infected the same cell (RTI) reassortment could occur and new variant of human virus bearing the avian virus HA may appear. Introduction: Swine influenza is a highly contagious respiratory disease in pigs caused by one of several swine influenza A viruses. Human cases of swine influenza A (H1N1) have been reported worldwide. In 2009, cases of influenza-like illness were first reported in Mexico on March 18; the outbreak was subsequently confirmed as swine influenza A. Viral Strain & Testing: Outbreaks of swine influenza are common in pigs year-round. Historically, when humans have become infected, it is a result of close contact with infected pigs. However, the current virus is a novel influenza A (H1N1) virus not previously identified in humans, and it spreads by human-to-human transmission. The WHO has raised its pandemic alert level for swine influenza to phase 6. Now we are in the post -pandemic period. Phase 4 is characterized by verified human-to-human transmission of an animal or humananimal influenza reassortant virus able to cause "community-level outbreaks." Transmission: Transmission of swine-origin influenza A(H1N1) occurs in ways similar to other influenza viruses. Seasonal human influenza viruses are spread from person to person primarily through large-particle respiratory droplet. Transmission via large-particle droplets requires close contact between source and recipient persons, because droplets do not remain suspended in the air and generally travel only a short distance (<1 meter) through the air . All respiratory secretions and bodily fluids (diarrheal stool) of 2009 (H1N1) cases should be considered potentially infectious. Incubation period: The estimated incubation period ranged from 1-7 days, and more likely 1-4 days. Symptoms: Manifestations of H1N1 influenza are similar to those of seasonal influenza. Patients present with symptoms of acute respiratory illness, including at least 2 of the following: Fever Cough Sore throat Body aches Headache Chills and fatigue Diarrhea and vomiting (possible). Symptoms of severe disease may include: Apnea Tachypnea Dyspnea Cyanosis Dehydration Altered mental status Extreme irritability Complications: 1. Exacerbation of underlying chronic medical conditions. 2. Upper respiratory tract disease (sinusitis, otitis media, croup). 3. Lower respiratory tract disease (pneumonia, bronchiolitis, status asthmaticus , ARDS ) . 4. Cardiac (myocarditis, pericarditis). 5. Musculoskeletal (myositis, rhabdomyolysis). 6. Neurologic (acute and post-infectious encephalopathy, encephalitis, febrile seizures, status epilepticus). 7. Toxic shock syndrome. 8. Secondary bacterial pneumonia with or without sepsis. Clinical deterioration is characterized by primary viral pneumonia, which destroys the lung tissue and does not respond to antibiotics, and the failure of multiple organs, including the heart, kidneys, and liver. These patients require management in intensive care units using therapies in addition to antivirals. Groups at high risk for complications: 1) Children less than 5 years old; 2) Persons aged 50 years or older; 3) Children and adolescents (aged 6 months–18 years) who are receiving long-term aspirin therapy and who might be at risk for experiencing Reye syndrome after influenza virus infection; 4) Pregnant women; 5) Adults and children who have chronic pulmonary, cardiovascular, hepatic, hematological, neurologic, neuromuscular, or metabolic disorders; 6) Adults and children who have immunosuppression (including immunosuppression caused by medications or by HIV); 7) Residents of nursing homes and other chronic-care facilities. 8) Obesity. Morbidity & Mortality: Swine influenza tends to cause high morbidity but low mortality rates (less than 1%.) Case Definitions for Infection with Swine-origin Influenza A (H1N1) Virus(S-OIV): A confirmed case of Swine flu infection is defined as a person with an acute febrile respiratory illness with laboratory confirmed S-OIV infection at CDC by one or more of the following tests: real-time RT-PCR viral culture A probable case of Swine flu infection is defined as a person with an acute febrile respiratory illness who is positive for influenza A, but negative for H1 and N1 by influenza RT-PCR. Treatment of Swine Flu: Vaccination : The 2009 influenza A (H1N1) monovalent vaccines are available as monovalent, inactivated influenza A virus vaccine (H1N1) for IM injection. and as monovalent live attenuated vaccine for intranasal administration. The immunization series consists of 2 doses for children younger than 10 years, consisting of an initial dose and a booster to be administered several weeks later. Adults and children 10 years and older will receive a single dose. Target populations recommended to receive the 2009 H1N1 vaccine include: Pregnant women, Household contacts and caregivers of children younger than 6 months, Healthcare and emergency medical services personnel. Conditions associated with higher risk of medical complications from influenza. A seasonal influenza vaccine will also need to be administered for the 2009/2010 influenza season. Treatment is largely supportive and consists of bedrest, increased fluid consumption, cough suppressants, and antipyretics and analgesics (eg, acetaminophen, nonsteroidal antiinflammatory drugs) for fever and myalgias. Severe cases may require intravenous hydration and other supportive measures. Antiviral agents may also be considered for treatment or prophylaxis. Patients should be encouraged to stay home if they become ill, to avoid close contact with people who are sick, to wash their hands often, and to avoid touching their eyes, nose, and mouth. Medications: The swine influenza A (H1N1) virus is susceptible to the prescription antiviral drugs oseltamivir and zanamivir. Initiation of antiviral agents within 48 hours of symptom onset, significantly decreases risk of pneumonia (a leading cause of death for both pandemic and seasonal influenza) and the need for hospitalization. The recommended duration of treatment is 5 days, but may be longer(10 days)in pediatrics and very severe cases. **Oseltamvir (Tamiflu): Oseltamivir inhibits neuraminidase, so it decreases the release of viruses from infected cells and, thus, viral spread. **Zanamivir (Relenza): Zanamivir inhibits neuraminidase .The preparation of zanamivir is in powder form for inhalation via the Diskhaler oral inhalation device. Individuals with asthma or other respiratory conditions that may decrease ability to inhale drug should be given oseltamivir.