* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download NutriStem hESC XF Serum
Survey
Document related concepts
Transcript
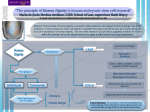
Biological Industries Israel Beit Haemek Ltd. Version 1.2 Page 1 of 6 Product Profile Product Name: Product Catalog Number Concentration: Unit Size Availability: Formulation: Optimal Storage Conditions: Stability: (Under Specified Handling & Storage) NutriStem hESC XF Serum-Free Medium (SFM) with Human Serum Albumin 05-100-1 1X (A) 500ml; (B)100ml Red-Colored Frozen Solution -20°C Please Refer To The Product Label Important Note! Please read the MSDS and Product Profile carefully in their entirety before using this material for possible safety precautions and potential hazards. Product Description: NutriStem hESC XF with Human Serum Albumin (HSA) , is a standardized serum-free medium for feeder-cell dependent and independent maintenance of Human Embryonic Stem Cells (hESC) in culture with no components of animal origin. It is a multi-purpose, ready-to-use defined formulation developed in collaboration with the Rambam Medical Center and The Technion Institute of Technology. NutriStem hESC XF has been specifically designed to maintain and expand undifferentiated hESC with the option of using the substrate Matrigel. As you are no doubt aware, that current hESC culture and expansion methods require the use of serum, mouse/human fibroblast feeder-layers, feeder-conditioned media or Matrigel. The major disadvantages of present culture methods are five-fold in nature and include some of the following: ♦ Very Labor-Intensive ♦ Hard to Scale ♦ Difficult To Maintain hESC's due to Growth Factor Fluctuations ♦ Medium with Animal Components ♦ Feeder Cells From an Animal Source Biological Industries, Kibbutz Beit Haemek 25115 Israel Telephone: 972-4-9960-595 Fax: 972-4-9968-896 Web Site: www.bioind.com Biological Industries Page 2 of 6 1 E-Mail: [email protected] Biological Industries' has developed NutriStem hESC XF as a breakthrough solution to these multi-faceted problems by enabling serum-free, animal component-free culture of hESC's on any type of feeder or matrix. Specifically, NutriStem hESC XF has been extensively tested and proven to maintain pluripotency in normal hESC lines. It has also been shown to support long-term growth of hESC's without any signs of karyotypic abnormalities and maintain the ability of these cells to differentiate into all three germ-line lineages. Note: Long-Term Growth of hESC on Matrigel requires the addition of Human Serum Albumin (HSA) to the medium(0.5% final). Human Embryonic Stem Cells (hESC's) grown in Biological Industries' NutriStem hESC XF may be definitively characterized in terms of: v v v v v v FACS Analysis of Pluripotent Marker Expression(PME) Immunofluorescence Analysis of PME Karyotype Analysis Maintenance of Differentiation Capabilities of hESC's Maintenance of Normal Morphology RT-PCR Analysis of Pluripotent Gene Expression Fibroblast Growth Factors (FGF's) This medium contains Fibroblast Growth Factors or FGF's which is typical of a family of growth factors that are involved in embryonic development, angiogenesis, proliferation of fibroblasts and wound healing(e.g. synthesis of connective tissue matrix and tissue remodeling). FGF's are heparin-binding proteins assuming a multi-functional role that interacts with cellsurface heparan sulfate proteoglycans, acting in concert by playing a key role in FGF signal transduction. Heparan sulfate proteoglycans (HSP's) are a major component of basement membranes. Some of the more common, general wide-ranging effects of FGF's include, but are not limited to: ♦ Endocrinal ♦ Mitogenic ♦ Morphological and ♦ Regulatory Modulation FGF's have also been referred to as growth factors with pluripotent functions owing to their wide-spectrum of activity on multiple, variegated cell types. Most are secreted proteins that bind heparin sulfates interacting in the formation of the extracellular matrix of tissues containing HSP's. Biological Industries, Kibbutz Beit Haemek 25115 Israel Telephone: 972-4-9960-595 Fax: 972-4-9968-896 Web Site: www.bioind.com Biological Industries Page 3 of 6 2 E-Mail: [email protected] Nutristem hESC XF contains Human Transferrin1 and bio-synthetically produced Human Recombinant Insulin (rHI) in trace amounts which is essential for long-term growth of various cells. It is commonly used as an additive to serum-free medium and fulfills some of the following functions including: ♦ Extends cell survival ♦ Enhances and stimulates the development and dynamic growth of cells ♦ Optimizes protein production time ♦ Role in Carbohydrate metabolism Human Transferrin1 Human Transferrin(hTr) is a glycoprotein found in blood plasma with the function of transporting iron to and from body cells. It is another of the key universal requirements in serum-free media. Transforming Growth Factor beta (TGF-β) The TGF-β family is part of a super-family of peptide structural proteins all encoded as large protein precursors found in both adult organisms and the developing embryo. Some of the major functions of this protein include, among many others: § Angiogenesis § Apoptosis § Cellular Differentiation § Cellular Homeostasis § Cellular Growth and Proliferation § Embryonic Development § Promotion of Wound-Healing The TGF-β system is a simple yet basic pathway that provides a signaling pathway for signals to pass from the extracellular environment to the nucleus via three different TGF-β molecules that all activate the same intracellular receptor. This family also plays a role in cancer, heart, disease, immunity and some chromosomal abnormalities. Human Serum Albumin (HSA)2 HSA plays a multi-faceted role in cell culture systems. One of the major functions is to bind, sequester and to stabilize a wide spectrum of important ions and small molecules. It is highly soluble and can bind anionic, cationic and neutral molecular structures. Albumin has a high affinity as well as secondary binding sites for many kinds and types of molecules. For example, it also exhibits anti-oxidant activity by binding with some of the following: • Bilirubin • Copper • Cysteine • Fatty Acids • Glutathione Biological Industries, Kibbutz Beit Haemek 25115 Israel Telephone: 972-4-9960-595 Fax: 972-4-9968-896 Web Site: www.bioind.com Biological Industries Page 4 of 6 3 E-Mail: [email protected] This Animal Component-Free medium and also obviously Serum-Free, contains all the necessary constituents of a ready-to-use formulation that includes a typical and wide variety of elements, among others: ♦ Amino Acids ♦ Glucose ♦ Inorganic Salts ♦ Fatty Acids ♦ Phenol Red ♦ Vitamins ♦ Trace Elements Nutristem hESC XF does not contain Antibiotics but does contain the dipeptide supplement, Alanyl-Glutamine. Some Predominant Characteristics of Nutristem hESC XF include: § Enables expansion of hESC's on Mouse(MEF) of Human-Feeder Layer(foreskin fibroblasts),Matrigel and on matrix prepared from the culture of normal human cells § Enables superior expansion of hESC's § Maintains differentiation capability of hESC's § Maintains pluripotency and normal morphology/karyotype of hESC's § Provides gene expression profiles comparable to classical media § Consistent Media Performance § Performance Tested § Sterile-Filtered(0.1µ) § Cell-Culture and Endotoxin-Tested Storage & Handling Precautions and Disclaimer: For in vitro research use only. The product should be stored at -20°C. The product should not be left in the light for prolonged periods as it is light-sensitive. When stored in the dark under ideal conditions, the product is stable until the expiry date. As with any other liquid media formulations, deterioration of liquid media may be recognized by any of the following characteristics, among others including: (a). color change, (b). presence of clumping/flocculent debris/ granulation/ particulates\ precipitates or sediments (c). insolubility,(d). and/or decrease in expected performance parameters. Any material described above should not be used and therefore discarded. Nutristem hESC XF contains bio-synthetically produced Human Recombinant FGF,TGF-β, Insulin and also Human Transferrin, and HSA in trace amounts. Biological Industries, Kibbutz Beit Haemek 25115 Israel Telephone: 972-4-9960-595 Fax: 972-4-9968-896 Web Site: www.bioind.com Biological Industries Page 5 of 6 4 E-Mail: [email protected] Instructions/Procedures: 1) Take a bottle of Nutristem hESC XF from proper storage conditions at -20°C and read the label and thaw to room temperature or 2-8°C. 2) Ensure that the cap of the bottle is tight. 3) Gently swirl the solution in the bottle to allow for uniformity. 4) Wipe the outside of the bottles with a disinfectant solution such as 70% ethanol. 5) Using aseptic/sterile technique under a laminar-flow culture hood, work according to established protocols. Quality Control: Test Appearance Growth Promotion Cell Line Endotoxins Growth Promotion Osmolality pH Sterility Specification Red-Colored Solution Test & Record hESC Test & Record Pass test 288-330mOsm/kg 7.1-7.5 Sterile Auxillary Products Product Name Catalog Number L-Glutamine Solution Alanyl-Glutamine Solution AF NutriStem hESC XF (w/o HSA) Bio-Pure Human Serum Albumin (HSA), 10% Serum-Free Cell Freezing Medium 03-020-1 03-022-1 05-102-1 05-720-1 05-065-1 Storage Temperature -20°C -20°C -20°C 2-8°C 2-8°C References: 1Human Transferrin, is manufactured under GMP conditions from Human Blood Plasma sub Fraction IV-1. It is important to emphasize that it is "For Research, Laboratory or Further Manufacturing Purposes Only. It is not intended For Human Use. The production process included heat treatment at 60°C for 10 Hours. It is a USA-sourced and approved product and plasma donors undergo a rigorous selection process as per USDA requirements. In addition, source material plasma units have been tested as per US FDA-approved or accepted testing for the presence of: • Anti (Human Immunodeficiency Virus) HIV-1/2 • Anti HIV • HBsAg • HCV,HBV,HAV,HIV-1, and Parvovirus B-19 by NAT (Minipools of 16v Donations) • Syphilis Biological Industries, Kibbutz Beit Haemek 25115 Israel Telephone: 972-4-9960-595 Fax: 972-4-9968-896 Web Site: www.bioind.com Biological Industries Page 6 of 6 5 E-Mail: [email protected] In addition, the plasma pool is also tested for the presence of: • Anti HIV-1/2 • Anti HIV • HBsAg • HCV by Nucleic Acid Testing(NAT) • HIV-1 by NAT • HBV by NAT • HAV by NAT(In-Process Only) • Parvovirus B-19 by NAT Human Serum Albumin, complies with the specifications of the manufacturer and the requirements stipulated by FDA approved tests. Each unit of plasma used in the manufacture of this product has been tested and found to be non-reactive for Hepatitis B Surface Antigen(HBsAg),antibody to HIV I-II and antiHCV. 3Biological Industries (BI )Specifications 4Darling, D.C. and Morgan S.J. Animal Cells: Culture and Media, John Wiley & Sons, New York, 1994 5Biological Industries (BI) Product Guide, "Human Cytogenetic Products" p.3. 2 Biological Industries, Kibbutz Beit Haemek 25115 Israel Telephone: 972-4-9960-595 Fax: 972-4-9968-896 Web Site: www.bioind.com 6 E-Mail: [email protected]