* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Nutrients - SBI3URHKing
Genetic code wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Human digestive system wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Biosynthesis wikipedia , lookup
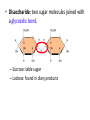
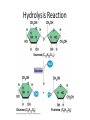
Nutrients & the Digestive System Four Stages of Processing Food 1. Ingestion: taking in and eating food 2. Digestion: breaking down food by mechanical (chewing) and chemical processes into smaller molecules 3. Absorption: transporting molecules from digestive system to the circulatory system 4. Elimination: removal of undigested solid waste from the body Macromolecules-Nutrients • Nutrients are required to perform life functions and obtain energy for survival 4 Macromoleules: • Carbohydrates • Lipids • Proteins • Nucleic Acids Micronutrients • Vitamins • Minerals We also need . . . • WATER • FIBRE Carbohydrates Carbohydrates • Carbohydrates are a family of organic molecules made up of carbon, hydrogen, and oxygen atoms. Some are small, simple molecules, while others form long polymers. • Carbohydrates have the general formula (CH2O)x • Primary function = short-term energy storage • Provides energy for cellular respiration Saccharide is derived from Greek meaning “sweet” • Monosaccharide: single sugar molecule – Glucose: sugar found in blood – Fructose: sugar found in fruit Glucose Fructose • Disaccharide: two sugar molecules joined with a glycosidic bond. – Sucrose: table sugar – Lactose: found in diary products • Polysaccharide: many sugars linked together – Starch: stores energy in plants – Glycogen: stores energy in animals – Cellulose: found in leaves of plants • Suffix “ose” is used when naming simple sugars (e.g., monosaccharides & disaccharides) Carbohydrates • Major source of energy • Monosaccharides provide quick energy because they do not have to be digested • Disaccharides and polysaccharides have to be digested • Cellulose cannot be digested, therefore it provides fibre or roughage in our diet Lipids Lipids • Examples: fats, waxes, steroids, phospholipids • Functions include: – Long-term energy storage – Fats cushion and protect organs and joints – Fat under the skin helps insulate the body – Steroids = hormones and cholesterol – Phospholipids = cell membrane Lipids • Why are lipids stored by the body? • Carbon-Hydrogen bonds are a very efficient way of storing energy • Lipids store more than twice the energy per gram than carbohydrates Proteins Proteins • Functions include: – When embedded into cell membranes : • help transport substances across membranes – Give cells support and shape (i.e., cytoskeleton) – Structure = muscle, skin, organs, hair – Enzymes: acts as catalysts to speed up chemical reactions in the cell Proteins • Structure – Proteins are made of long chains of amino acids – About 22 different Amino Acids – 8 Amino Acids are considered ESSENTIAL and must be obtained from the food we eat Amino Acids • Amino Acids are joined together peptide bonds • Therefore, chains of amino acids are called polypeptides • A single protein may be made up of several polypeptide chains Breaking Down Macromolecules: Enzymes • Before the body can use macromolecules, they need to be broken down into molecules small enough to be absorbed by the small intestine. • Enzymes are used to speed up the process of chemically digesting macromolecules! • This chemical break down is called hydrolysis Hydrolysis Reaction • A disaccharide is split into two monosaccharides by a hydrolysis reaction where one water molecule is added Hydrolysis Reaction Common Enzymes • Amylase: – Produced in the salivary glands – Released into the mouth – Breaks down polysaccharides • Pancreatic lipase: – Produced in the pancreas – Released into the small intestines – Breaks down lipids Common Enzymes • Pepsin: – Produced in stomach glands – Released into the stomach – Breaks down proteins Vitamins • Cannot be used for energy • Organic substances (C, H, O, N + other atoms) – Vitamin A: vision – Vitamin C: boosts immune system • May act as co-enzymes Minerals • Cannot be used for energy • Inorganic substances: Do not contain Carbon • For example: – Iron in hemoglobin of red blood cells – Calcium in bones and teeth – Sodium in muscle and nerve cells – Potassium to regulate heart beat and nerve signals – Iodine for thyroid (see salt) – Trace elements (e.g., zinc, selenium) Water • One can go without food for several weeks, but can only go without water for a few days • Water is used for: – Transporting nutrients – Eliminating waste – Lubricating joints – Forming bodily fluids; blood & mucus – Regulating body temerature; sweating Fibre • Roughage – cellulose (cannot be digested) • Functions by aiding digestion – Keeps digested food moist and soft (thereby helping to prevent constipation) – Physically scrubs digestive tract (cleans and helps to remove toxins) Homework • Refer to Section 10.1 • Answer questions on page 410 • # 3, 5-7,9,10, 11,12