* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Physcal Chemistry ERT 108 semester II 2010/2011
Eigenstate thermalization hypothesis wikipedia , lookup
Thermal radiation wikipedia , lookup
Glass transition wikipedia , lookup
Bose–Einstein condensate wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Thermodynamic equilibrium wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Temperature wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Van der Waals equation wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Heat transfer physics wikipedia , lookup
State of matter wikipedia , lookup
Equation of state wikipedia , lookup
Transition state theory wikipedia , lookup
Degenerate matter wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermodynamics wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
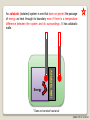
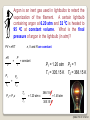
PRT 140 PHYSICAL CHEMISTRY PROGRAMME INDUSTRIAL CHEMICAL PROCESS SEM 1 2013/2014 INTRODUCTION TO PHYSICAL CHEMISTRY BY PN ROZAINI ABDULLAH SCHOOL OF BIOPROSES ENGINEERING ©RBA FTK RY 20 2013 LEARNING OUTCOME: Ability to explain the phenomena, basic concepts, laws and principles in physical chemistry. In this chapter you may be able to: ©RBA FTK RY 20 2013 What is Physical Chemistry? Physical chemistry is a study of the physical basis of phenomena related to the chemical composition and structure of substances. OR “It is the science of explaining physical phenomenon in chemical terms” What is Chemical Systems? A chemical system can be studied from either a microscopic or a macroscopic viewpoint. ©RBA FTK RY 20 2013 static phenomena macroscopic phenomena equilibrium in macroscopic systems THERMODYNAMICS ELECTROCHEMISTRY dynamic phenomena change of concentration as a function of time (macroscopic) KINETICS (ELECTROCHEMISTRY) STATISTICAL THEORY OF MATTER microscopic phenomena stationary states of particles (atoms, molecules, electrons, nuclei) e.g. during translation, rotation, vibration • bond breakage and formation • transitions between quantum states STRUCTURE OF MATTER CHEMICAL BOND STRUCTURE OF MATTER (microscopic) KINETICS CHEMICAL BOND ©RBA FTK RY 20 2013 What is Thermodynamic? Thermodynamic is a microscopic science that studies the interrelationships of the various equilibrium properties of a system and the changes in equilibrium properties. Thermodynamic is the study of heat, work, energy and the changes they produce in the state of system. SURROUNDING SYSTEM Everything outside surroundings. a system is called The macroscopic part of the universe under study in thermodynamics is called the system. The boundary or wall separates a system from it surroundings. ©RBA FTK RY 20 2013 Open System Definition: An open system can exchange matter and energy with its surroundings. How does it work? Example: Surrounding Matter System Energy ©RBA FTK RY 20 2013 Closed System Definition: A closed system can exchange energy with its surroundings, but it cannot exchange matter. How does it work? Example: Surrounding Matter System Energy ©RBA FTK RY 20 2013 Isolated System Definition: An isolated system can exchange neither energy nor matter with its surroundings. How does it work? Example: Surrounding Matter System Energy ©RBA FTK RY 20 2013 Walls A system may be separated from its surroundings by various kinds of wall: A B A Wall B 1. A wall can be rigid or non rigid (movable). 2. A wall may be permeable or impermeable (allows no matter to pass through it.) 3. A wall may be adiabatic or nonadiabatic. ©RBA FTK RY 20 2013 An adiabatic (isolated) system is one that does not permit the passage of energy as heat through its boundary even if there is a temperature difference between the system and its surroundings. It has adiabatic walls. Adiabatic Wall Energy * Does not conduct heat at all ©RBA FTK RY 20 2013 A nonadiabatic or diathermic (closed) system is one that allows energy to escape as heat through its boundary if there is a difference in temperature between the system and its surroundings. It has diathermic walls. Diathermic Wall Energy * Does conduct heat ©RBA FTK RY 20 2013 Equilibrium Isolated system is in equilibrium when its macroscopic properties remain constant with time. (a) The system macroscopic properties remain constant with time Non-isolated system is in equilibrium when the following conditions hold: (b) Removal of the system from contact with its surroundings causes no change in the properties of the system If (a) hold but (b) does not hold – the system is in a steady state. ©RBA FTK RY 20 2013 Types of Equilibrium 1. Mechanical equilibrium • No unbalanced forces act on or within the system; hence the system undergoes no acceleration, and there is no turbulence within the system. 2. Material equilibrium • No net chemical reactions are occurring in the system, nor is there any net transfer of matter from one part of the system to another or between the system and its surroundings; the concentrations of the chemical species in the various parts of the system are constant in time. 3. Thermal equilibrium between a system and its surroundings • There must be no change in the properties of the system or surroundings when they are separated by a thermally conducting wall. ©RBA FTK RY 20 2013 Thermodynamic Properties Extensive Variables Is one whose value is equal to the sum of its values for the parts of the system. Thus, if we divide a system into parts, the mass of the system is the sum of the masses of the parts; mass is an extensive property. Intensive Variables Is one whose value does not depend on the size of the system, provided the system remains of macroscopic. Examples: pressure, density Examples: mass, volume, energy ©RBA FTK RY 20 2013 Homogeneous System: Each of intensive macroscopic property is constant throughout a system. Heterogeneous System: A system compose of 2 or more phases. Phase: Homogeneous part of a (possibly) heterogeneous system. Equilibrium condition: The macroscopic properties do not change without external influence. The system returns to equilibrium after a transient perturbation. In general exists only a single true equilibrium state. ©RBA FTK RY 20 2013 Temperature “2 system in thermal equilibrium with each other have the same temperature “ A + B A B or “2 system not in thermal equilibrium have different temperature” A + B A B The zeroth law of thermodynamics Allows us to assert the existence of temperature as a state function ©RBA FTK RY 20 2013 The Zeroth Law of thermodynamics: If A is in thermal equilibrium with B, and B is in thermal equilibrium with C, than C is also in thermal equilibrium with A. All these systems have a common property: the same temperature. ©RBA FTK RY 20 2013 The Mole The ratio of the average mass of an atom of an element to the mass of some chosen standard. The Relative Atomic Mass of a chemical element gives us an idea of how heavy it feels (the force it makes when gravity pulls on it). The relative masses of atoms are measured using an instrument called a mass spectrometer. Look at the periodic table, the number at the bottom of the symbol is the Relative Atomic Mass (Ar ): ©RBA FTK RY 20 2013 Relative Molecular Mass, Mr Most atoms exist in molecules. To work out the Relative Molecular Mass, simply add up the Relative Atomic Masses of each atom in the molecule: A relative molecular mass can be calculated easily by adding together the relative atomic masses of the constituent atoms. For example, Nitrate, NO3, has a Mr of 62 g/mol (Try it!). ©RBA FTK RY 20 2013 Gram Molecular Mass Molecular mass expressed in grams is numerically equal to gram molecular mass of the substance. Molecular mass of O2 = 32Gram Calculation of Molecular Mass Molecular mass is equal to sum of the atomic masses of all atoms present in one molecule of the substance. Example: – H2O Mass of H atom = 18g – NaCl = 58.44g ©RBA FTK RY 20 2013 Avogadro’s Number “The number of 12C atoms in exactly 12 g of 12C” Avogadro's number = 6.02 x 1023 Atomic Mass or Molecular Mass The average mass of an atom or molecule Mole A mole of some substances is define as an amount of that substance which contains Avogadro’s Number of elementary entities. E.g: 1 mole of hydrogen atoms contain 6.02 x 1023 H ©RBA FTK RY 20 2013 Molar Mass = the mass of substance i in a sample , = the number of moles of i in the sample Mole fraction ©RBA FTK RY 20 2013 Ideal Gases Boyle’s Law Boyle investigated the relationship between pressure & volume of gases. P a 1/V P x V = constant P1 x V1 = P2 x V2 Constant temperature Constant amount of gas ©RBA FTK RY 20 2013 A sample of nitrogen gas occupies a volume of 800 mL at a pressure of 726 mmHg. What is the pressure of the gas (in mmHg) if the volume is reduced at constant temperature to 404 mL? P1 x V1 = P2 x V2 P1 = 726 mmHg P2 = ? V1 = 800 mL V2 = 404 mL P1 x V1 P2 = = V2 726 mmHg x 800 mL = 1437.62 mmHg 404 mL Convert 1437.62 mmHg to atm and pascal? ©RBA FTK RY 20 2013 Charles’ & Gay-Lussac’s Law Measured the thermal expansion of gases and found a linear increase with temperature. VaT V = constant x T, at constant n, p p= constant x T, at constant n, V V1/T1 = V2/T2 Temperature must be in Kelvin T (K) = t (0C) + 273.15 ©RBA FTK RY 20 2013 A sample of carbon monoxide gas occupies 4.2 L at 234 0C. At what temperature will the gas occupy a volume of 2.0 L if the pressure remains constant? V1/T1 = V2/T2 V1 = 4.20 L V2 = 2.0 L T1 =507.15 K V2 x T1 T2 = = V1 T2 = ? 2.0 L x 507.15 K = 241.5 K 4.2 L ©RBA FTK RY 20 2013 Avogadro’s Law V a number of moles (n) V = constant x n V1/n1 = V2/n2 Constant temperature Constant pressure ©RBA FTK RY 20 2013 Ideal Gas Equation Boyle’s law: V a 1 (at constant n and T) P Charles’ law: V a T (at constant n and P) Avogadro’s law: V a n (at constant P and T) V a nT P V = constant x nT P = R nT P R is the gas constant = 8.314 47 JK-1mol-1 PV = nRT ©RBA FTK RY 20 2013 Ideal Gas Mixture Partial Pressure , ©RBA FTK RY 20 2013 The conditions 0 0C and 1 atm are called standard temperature and pressure (STP). Experiments show that at STP, 1 mole of an ideal gas occupies 22.414 L. PV = nRT R= PV nT (1 atm)(22.414L) = (1 mol)(273.15 K) R = 0.082057 L • atm / (mol • K) ©RBA FTK RY 20 2013 What is the volume (in liters) occupied by 64.9 g of HCl at STP? T = 0 0C = 273.15 K P = 1 atm PV = nRT V= nRT P 1.78 mol x 0.0821 V= 1 mol HCl n = 64.9 g x = 1.78 mol 36.45 g HCl L•atm x 273.15 K mol•K 1 atm V = 39.9 L ©RBA FTK RY 20 2013 Argon is an inert gas used in lightbulbs to retard the vaporization of the filament. A certain lightbulb containing argon at 6.20 atm and 32 0C is heated to 95 0C at constant volume. What is the final pressure of argon in the lightbulb (in atm)? n, V and R are constant PV = nRT nR P = T V P1 T1 = constant P2 = P1 = 1.20 atm T1 = 305.15 K P2 = ? T2 = 368.15 K T2 P2 = P1 x T2 T1 = 1.20 atm x 368.15 K = 1.45 atm 305.15 K ©RBA FTK RY 20 2013 Dalton’s Law of Partial Pressures The pressure exerted by a mixture of gasses is the sum of the pressure that each one would exert if it occupied the container alone. V and T are constant P1 P2 Ptotal = P1 + P2 ©RBA FTK RY 20 2013 Consider a case in which two gases, A and B, are in a container of volume V. PA = PB = nART V nBRT V nA is the number of moles of A nB is the number of moles of B nB nA PT = PA + PB PA = XA PT XA = nA + nB XB = nA + nB PB = XB PT Pi = X i PT ©RBA FTK RY 20 2013 A sample of natural gas contains 8.24 moles of CH4, 0.421 moles of C2H6, and 0.116 moles of C3H8. If the total pressure of the gases is 1.37 atm, what is the partial pressure of propane (C3H8)? Pi = Xi PT PT = 1.37 atm 0.116 Xpropane = = 0.0132 8.24 + 0.421 + 0.116 Ppropane = 0.0132 x 1.37 atm = 0.0181 atm ©RBA FTK RY 20 2013 The van der Waals Equation R= gas constant T = Temperature P = pressure b & a = van der waals coeeficient Vm = V/n , volume ©RBA FTK RY 20 2013 Calculate the pressure (unit in atm) exerted by 2.0 mol C2H6 at 273.15 K in 22.414 dm3 behaving as (i) Perfect gas (ii) a van der Waals gas. C2H6 C6H6 CH4 a (atm.dm6.mol-2) b (10-2 dm3.mol-1) 5.507 18.57 2.273 6.51 11.93 4.31 ©RBA FTK RY 20 2013 Thank you…. ©RBA FTK RY 20 2013