* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CH2=CH2
Survey
Document related concepts
Transcript
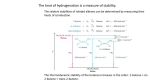
هبة حيدر ابراهيم:مدرس Alkenes Structre and synthesis of alkenes 2.1 Introduction Alkenes are hydrocarbons with carbon-carbon double bonds alkene are sometime called olefins a term derived from olifiantgas,meaning (oil-forming gas). CH2=CH2 Ethylene(ethane) 2.2 The orbital description of the alkene double bond In a lewisstructure,the double of an alkene is represented by two pairs of electrons between the carbon atoms. 2.2A The sigma bond framework Each of the carbon-hydrogen bonds is formed by overlap of on sp2 hybrid on carbon with the 1s orbital of a hydrogen atom. The C-H bond length in ethylene (1.08 A0) is slightly shorter than the C-H bond in ethane (1.09 A0) ,because the sp2 orbital in ethylene has more s character (one-third s) than an sp3orbital (one-fourth s). The s orbtal is closer to the nucleus than the p orbital, contributing to shorter bond. 2.2B The pi bond Two more electrons must go into the carbon-carbon bonding region .Each carbon atom still has an additional unhybradized p orbital ,and these overlap to form a pi bonding molecular orbital. 2.3 Elements of unsaturation Alkenes are called unsaturated because they react with hydrogen in the presence of catalyst.Theproduct,analkane,iscalled saturated because it cannot react with any more hydrogen . CH3-CH2-CH3 CH3-CH=CH2 CH2=C(CH3)2 Propane,C3H8 propane C3H6unsaturated isobutylene saturated 2.4 Nomenclature of alkene Simple alkenes are named much like alkanes, using the root name of the longest chain containing the double bond. The ending is changed from –ane to –ene. For example , (ethane) becomes (ethane) ,(propane) becomes (propene) and (cyclohexane) becomes (cyclohexene) . 2.5 Commercial Importance of alkenes. Because the carbone-carbon double bond is readily converted to other functional groups, alkenes are important intermediates in the synthesis of drugs, pesticides, and other valuble chemical. 2.6A Heat of Hydrogenation 2.6B Energy differences in cis-trans isomers The trans isomers are generally more stable than the corresponding cis isomers. This trend seems reasonable because the alkyl substituents are separated farther in the trans isomers than they are in the cis isomer. 2.7 A-Boiling points and densities The boiling points of alkene increase smothly with molecular weight. As with alkene, increase branching leads to greater volatility and lower boiling points. For example, isobutylene has a boiling point of -70C ,which is lower than the boiling point of any of the unbranchedbutenes. name structure carbone B.P. 0 C Density(g/cm3) Ethane CH2=CH2 2 -104 - Propene CH3CH=CH2 3 -47 0.52 4 -7 0.59 CH3CH2CH=CH2 4 -6 0.59 Isobutylene (CH3)2C=CH2 1-butene B – polarity Like alkenes, alkene are relatively nonpolar. They are insoluble in water but soluble in nonpolar solvents such as hexane, gasoline, halogenated solvents, and ethers. A alkene tend to be slightly more polar than alkanes, however, because the more weakly held electrons in the pi bond are more polarizable. 2.8 Alkene synthesis by Elimination of alkyl halides A- Dehydrohalogenation by the E2 mechanism Second-order elimination is a reliable synthetic reaction. B- Dehydrohalogenation by the E1 mechanism First-order dehydrohalogenation usually takes place in a good ionizing solvent (such as an alcohol or water). 2.9 Alkene synthesis by dehydration of alcohols Dehydration of alcohols is one of the best methods for the synthesis of alkene. The worddehydrationliterall means the removal of water.