* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Fertilisers
Process chemistry wikipedia , lookup
Catalytic reforming wikipedia , lookup
Biochemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Acid–base reaction wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Soil contamination wikipedia , lookup
List of phenyltropanes wikipedia , lookup
Atomic theory wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Constructed wetland wikipedia , lookup
Isotope analysis wikipedia , lookup
Metalloprotein wikipedia , lookup
Nitrocellulose wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
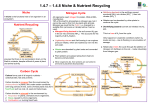
Why do plants need fertilisers? 1. Which 3 elements did mum tell Davy are needed by plants? Phosphorus is needed to help build a large healthy root system. Carrots, potatoes, turnips etc need fertilisers rich in phosphorus Lack of phosphorus causes small seeds and fruit and slow growth Nitrogen is needed to build proteins. Proteins are used to build large leaves and tall plants. Potassium helps produce protein and sugars, and is needed for healthy flowers e.g. orange blossom and large tasty fruit. Lettuces, cabbages, wheat and spinach need fertilisers high in nitrogen Tomatoes, apples, strawberries, bananas and pear trees need fertilisers high in potassium Lack of nitrogen causes smaller crops with yellowish leaves and smaller roots Lack of potassium causes smaller plants with less resistance to disease, droughts and frost 2. Copy and complete the table Element Why needed by the plant Symptom of lack of element Soil contains many elements. Main soil nutrients. Name silicon Aluminium iron calcium sodium magnesium % 25 10 5 4 3 2 3. Plot a bar graph showing soil elements a. Which 3 elements are the most common in soil ? b. Which element is the least common? c. Which 3 elements do plants need? d. Suggest why farmers need to add fertilisers to the soil. COPY The essential elements in fertilisers must be taken up by the plant roots so the fertiliser must be soluble Use your data booklet to help you to copy and complete the table Chemical calcium phosphate potassium nitrate magnesium nitrate potassium phosphate essential element soluble/ insoluble suitable as fertiliser? Natural replacement of soil nutrients There are 4 main ways nutrients get replaced naturally. 1. Decay and excretion In nature, nutrients are returned to the soil when dead ......................... and plants decay or when .......................... excrete waste. Harvesting crops and eating animals stops …………….getting back into the soil naturally. Composting allows waste plant materials to be used as fertiliser. Slurry pits store …………..to be sprayed on fields. 2. Root nodules Some plants such as peas, beans and clover have root nodules. These plants are known as ‘legumes’ The root nodules contain bacteria which can change nitrogen from the air into soluble nitrogen (nitrate) Crop rotation systems use these plants to replace nitrogen in soil. Different crops are planted in the fields each year. One year a field may grow wheat, the next year it may be used for growing peas and the following year it may be used for grazing. Chemistry counts p 250 shows root nodules a. What are ‘legumes? Name 3 legumes. b. How do legumes help to improve the soil? c. Explain how crop rotation would help maintain a high level of nitrate in the soil. d. What are the disadvantages of crop rotation? 3. Lightning storms Watch the Demo of sparking Air 80% of the air is nitrogen, which is a very unreactive element because it has a triple covalent bond. During a lightening storm, the enormous amount of electrical energy is able to split the triple bond, enabling the nitrogen to react with oxygen to form nitrogen dioxide. This gas dissolves in the water vapour of the air to form nitric acid which falls to earth in the rain to provide nitrate in the soil. a. Why is nitrogen so unreactive? b. Write a word equation to show the reaction of oxygen and nitrogen. c. What colour was the nitrogen dioxide gas that formed? d. What colour did the indicator go when the water was added to the flask? e. What have you learned about the pH of the solution made when nitrogen dioxide dissolves in water? f. Write the formulae of nitrogen dioxide, nitric acid and potassium nitrate. g. Nitrogen dioxide is also produced by car engines because of the high temperature spark that ignites the fuel. What device is used to remove nitrogen dioxide from car exhaust systems? h. Why is nitrogen dioxide removed from exhaust emissions? ARTIFICIAL FERTILSERS It is inefficient to use the sparking air method to manufacture nitrate fertilisers on a large scale because of the enormous energy costs. Towards the end of the nineteenth century, the demand for food grew faster than the supply. A new, artificial source of nitrogen was needed. The challenge was to find a way to get unreactive nitrogen to make useful compounds. The solution was discovered by Fritz Haber who developed the 'Haber Process' to make ammonia NH3 Since then, the amount of fertiliser used has increased rapidly. USE OF FETILISERS Year 1875 1940 1950 1980 World population 1 billion 2 billion 2.8 billion 5 billion Potassium fertiliser ( tonnes) 2, 500 63,000 198, 000 320,000 1. Plot two line graphs showing how the use of fertilisers and the population of the world have changed over the last 125 years. 2. Why has the use of Fertiliser increased over the last 125 years? PROPERTIES OF AMMONIA 1. The Fountain experiment. a. Draw a diagram of the apparatus. b. What two properties of ammonia does the experiment show? c. A solution of ammonia in water is called aqueous ammonia or ammonium hydroxide. Copy and complete the equations ammonia + _________ → ___________ ____________ ________(g) + H2O (l) → NH4 Cl (aq) 2. Laboratory Preparation of ammonia Ammonia is produced by heating an ammonium salt with soda lime which is a mixture of calcium hydroxide and sodium hydroxide. a. Copy the equation below and write a word equation for the reaction. NH4 Cl + NaOH → NaCl + H2O + NH3 b. Draw a labelled diagram of the apparatus c. What was the effect of ammonia on pH paper? d. What colour is ammonia? e. Chemists say that ammonia has a ‘pungent’ smell. How would you describe it? AMMOMNIA AND FERTILISERS Many fertilisers are made from ammonia. Ammonia will neutralise acids to make ammonium salts which are used as fertilisers. e..g ammonia + nitric acid → NH3 +HNO3 → ammonium nitrate + water NH4 (NO3 ) + H2O 1. Write word equations to show how ammonia could be used to make a. ammonium sulphate b. ammonium phosphate ( you need phosphoric acid) 2. CREDIT. Write balanced chemical equations for the reactions in question 1 Your teacher may show you how to make ammonium sulphate fertiliser. THE HABER PROCESS Copy and complete. The DVD AND CD-ROM will help you The Haber Process uses hydrogen from ____________ and ___________ from the air to make ammonia. The reaction uses an ________ catalyst to it to go faster at lower ________________. A ___________speeds up a reaction but is left unchanged at the end. The Haber Process uses a temperature of ________ and a pressure of __________ atmospheres More _________ would be produced at a lower temperature but the reaction would happen too slowly to be economic. The reaction is reversible. This is shown in the equation by the use of the __________ symbol Hydrogen + __________ 3H2 + N2 ammonia 2 NH3 Use the CD ROM or other resources to find out about the many uses of ammonia THE PRODUCTION OF NITRIC ACID – THE OSTWALD PROCESS Nitric acid can be made from nitrogen dioxide but, as we have seen it is expensive to make nitrogen dioxide by direct combination of nitrogen and oxygen because of the high temperatures needed. An alternative way to make nitrogen dioxide is by the catalytic oxidation of ammonia using a platinum catalyst in the Ostwald Process. Ammonia is oxidised to nitrogen monoxide which reacts with the air to give nitrogen dioxide. The nitrogen dioxide dissolves in water in the presence of air to make nitric acid. Nitric acid is used to make nitrate fertilisers and explosives. Watch the demonstration a. What is the main product of the Ostwald Process and how is it produced? b Which catalyst is used? c. Why does the catalyst stay at a high enough temperature once the reaction has started? d. What evidence was there that nitrogen dioxide had formed? e. Complete the word equations to show the stages in the Ostwald Process ___________ + oxygen → nitrogen monoxide nitrogen monoxide + ___________ → nitrogen dioxide nitrogen dioxide + _______ + oxygen → nitric acid PROBLEM SOLVING Key : MAKING AMMONIUM NITRATE FERTISLISER chemical Process Use the chemicals and processes shown below to create a flowchart showing how to produce ammonium nitrate fertiliser oxygen ammonia nitric acid hydrogen water nitrogen dioxide Haber Process (iron catalyst) nitrogen Neutralisation ammonium nitrate Ostwald Process (platinum catalyst) CREDIT: WHICH FERTILISER TO USE? Different crops need different amounts of nitrogen, phosphorus and potassium. Grass, which farmers use for animal feed, needs a lot of nitrogen, so ammonium nitrate ( NH4 NO3 ), a nitrogen only fertiliser would be suitable. Wheat requires all three essential elements so a NPK compound fertiliser is used. NPK fertilisers are usually a mixture of ammonium nitrate (NH4 NO3 ) ammonium phosphate(( NH4)3 PO4 ) and potassium chloride (KCl). Some examples of brand name fertilisers are listed in the table along with their NPK percentages FERTILISER NITRAPRIL USED FOR GRASS ICI NUMBER 1 POTATOES, WHEAT POTATOES WHEAT ICI NUMBER 7 PERCENTAGE N P 35 0 K 0 15 15 20 17 17 17 CALCULATING THE PERCENTAGE OF N, P AND K 1. Work out the gram formula mass of the compound e.g. ( NH4)3 PO4 ( 14 + 1+1+1+1) x 3 + 31 + (16 x 4 ) = 149 So 1 mole is 149g 2. To work out the percentage of nitrogen Mass of nitrogen in 1 mole is 14 x 3 = 42g % N = 42 x 100 = 28.2% 149 3. To work out the percentage of phosphorus Mass of phosphorus in 1 mole is 31g % P = 31 x 100 = 20.8% 149 How do artificial fertilisers compare to manure? Manure is 0.5% N 0.2 % P and 0.4% K 1. Calculate the percentage of nitrogen in a. b. c. d. ammonia ammonium nitrate ( nitram) Ammonium hydrogen phosphate . ( NH4)2 HPO4 urea CO ( NH2)2 How do their percentages of nitrogen compare to manure? 2. Calculate the percentage of phosphorus in a. calcium phosphate Ca 3 (PO4) 2 b. calcium superphosphate Ca (H 2PO4) 2 How do their percentages of P compare to manure? Why is the name ‘superphosphate’ used? PROBLEMS WITH FERTILISERS Because nitrate compounds used in fertilisers are so soluble they easily wash off into rivers and lochs. High levels of nitrates in lochs and slow moving rivers cause rapid growth in algae which can harm fish stocks and water plants. No –one is sure if the higher levels of nitrate in drinking water could cause harm to our health. There may be some harm to bottle fed babies although there is no direct evidence of this. Nitrates may break down to cancer causing compounds in the stomach. Possible solutions to the problem Only use fertiliser when it is most needed e.g. when the crops are growing fastest. Use slow dissolving fertiliser pellets Use less fertiliser. QUESTIONS 1. Why do nitrate fertilisers wash off easily into rivers and lochs? 2. What happens to lochs and rivers with high levels of nitrate? 3. What problems may be caused by increased levels of nitrate in drinking water? 4. Give 3 solutions to the nitrate problem. LEARNING OUTCOMES FOR TOPIC 14, FERTILISERS Traffic light the learning outcomes at the end of the Topic GENERAL At the end of the Topic you should know o Why there has been an increase in demand for fertilisers o Which three elements are essential to healthy plant growth o Why legumes are important to soil fertility o Three other ways in which nitrogen is replaced in the soil naturally o Why nitrogen can react during thunderstorms or in a car engine o Why fertilisers are important o Why fertilisers have to be soluble compounds o Why nitrogen is unreactive o The conditions that are used in the Haber Process and the name and formula of the compound that is formed in the Haber Process o Why ammonia is an important industrial product o The properties of ammonia o The main product of the Ostwald Process o Which fertiliser is made from nitric acid and ammonia o How nitrogen is recycled in the ‘nitrogen cycle’ CREDIT o Why different crops need different types of fertiliser o How to calculate the % of N, P or K in different fertilisers o Why chemical fertilisers are more expensive than organic fertilisers o Why the Haber Process is carried out at a moderately high temperature o Why not all the nitrogen and hydrogen are converted to ammonia in the Haber Process o How to prepare ammonia in the laboratory o Why there is no need to heat the catalyst in the Ostwald Process NITROGEN CYCLE The nitrogen cycle is the term used to describe how nitrogen is recycled. Plants use nitrogen from the soil to make protein. Animals use the nitrogen from plants to make animal proteins. Animals excrete ammonium compounds in urine and feaces. When plants and animals die, the nitrogen in returned to the soil as their bodies decay. Some bacteria in the soil break down nitrates into atmospheric nitrogen. Atmospheric nitrogen Haber Process Lightning storms Denitrifying bacteria in the soil root nodules in legumes nitrates in the soil death and decay proteins in plants excretion, death and decay protein in animals