* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download j=1/2
Neutron magnetic moment wikipedia , lookup
Earth's magnetic field wikipedia , lookup
Electromotive force wikipedia , lookup
Magnetotellurics wikipedia , lookup
Magnetoreception wikipedia , lookup
Electromagnet wikipedia , lookup
Electromagnetism wikipedia , lookup
Giant magnetoresistance wikipedia , lookup
Magnetohydrodynamics wikipedia , lookup
Electron paramagnetic resonance wikipedia , lookup
Multiferroics wikipedia , lookup
History of geomagnetism wikipedia , lookup
Electromagnetic field wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
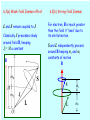
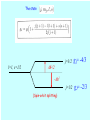
PHYS 30101 Quantum Mechanics Lecture 20 Dr Jon Billowes Nuclear Physics Group (Schuster Building, room 4.10) [email protected] These slides at: http://nuclear.ph.man.ac.uk/~jb/phys30101 6. The hydrogen atom revisited - Reminder of eigenfunctions, eigenvalues and quantum numbers n, l, ml of hydrogen atom. 6.1 Spin-orbit coupling and the fine structure. 6.2 Zeeman effect for single electron atoms in (a) a weak magnetic field (b) a strong magnetic field 6.3 Spin in magnetic field: QM and classical descriptions RECAP: 6. 1 Spin-orbit coupling and “fine structure” Classically an electron orbiting a nucleus of charge Ze “sees” the nucleus in orbit around it (a current loop) which produces a field at the electron of Putting I= Ze/T where T is period of orbit (obtained from the classical angular momentum expression L=meωr2 ) we get: Electron magnetic moment is in direction of its intrinsic spin: Thus interaction energy and corresponding Hamiltonian can be written (relativistic effect of “Thomas precession”) The shift in energy of a state is the eigenvalue of the spin-orbit Hamiltonian: j=3/2 (4 states) l=1, s=1/2 ml=+1, 0, -1 ms=+1/2, -1/2 (6 states) Aħ2/2 -Aħ2 j=1/2 (2 states) The energy centroid is unchanged: 4 X A/2 = 2 X A 6.2(a) Weak-field Zeeman effect L and S remain coupled to J. Classically J precesses slowly around field B, keeping Jz= M a constant 6.2(b) Strong-field Zeeman For electron, B is much greater than the field it ”sees” due to its orbital motion. S and L independently precess around B keeping ms and ml constants of motion B B S L S ms L ml Landé g-factor The state l=1, s=1/2 j=3/2 gJ= -4/3 j=1/2 gJ= -2/3 Aħ2/2 -Aħ2 (Spin-orbit splitting) Zeeman structure Strong field for l = 1, s = 1/2 orbital Weak field (-1,+1/2) (+1,-1/2)