* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PDF
Survey
Document related concepts
Transcript
/ . Embryo I. exp. Morph. Vol. 52, pp. 105-113, 1979
Printed in Great Britain © Company of Biologists Limited 1979
105
Evidence for a positional memory in the
development of the chick wing bud
By J. C. SMITH 1
From the Department of Biology as Applied to Medicine,
The Middlesex Hospital Medical School, London
SUMMARY
Grafts of quail zones of polarizing activity (ZPA), treated with 10000 rad y-radiation, tend
to remain at the base of a limb. Their signalling ability is not passed on to more distal tissue,
but the limb goes on to produce a reduplication. This suggests that the effect of a ZPA can
be remembered in its absence, and explains why a normal limb can develop if its ZPA is
removed.
INTRODUCTION
Pattern formation in the developing chick limb bud can be viewed in terms of
positional information. Cells are assigned positional values in a three-dimensional coordinate system, and interpretation of these positional values leads to
the appropriate cell behaviour (Wolpert, 1969; Wolpert, Lewis & Summerbell,
1975). For the antero-posterior axis of the chick limb, it appears that specification of positional information is due to a group of cells at the posterior margin
of the limb - the zone of polarizing activity (ZPA). If an additional ZPA is
grafted to the anterior margin of a wing bud, the wing that develops has mirrorimage symmetry about its long axis (Saunders & Gasseling, 1968; MacCabe,
Gasseling & Saunders, 1973; Summerbell, 1974a; Tickle, Summerbell &
Wolpert, 1975; Fallon & Crosby, 1977; Summerbell & Tickle, 1977). By
grafting an additional ZPA to different positions along the antero-posterior
axis, Tickle et al. (1975) found that structures form according to their distance
from the ZPA. They suggested that positional information is specified by the
concentration of a diffusible morphogen produced by the ZPA. In support of
this Smith, Tickle & Wolpert (1978) have been able progressively to attenuate
the signal from the ZPA by treating it with increasing doses of y-radiation, and
evidence for a gradient of a morphogen comes from the in vitro bioassay of
MacCabe & Parker (1975, 1976).
However, some doubt has been expressed about the role of the ZPA in limb
development (see Saunders, 1977, for a discussion). One difficulty is that
1
Author's address: Department of Biology as Applied to Medicine, The Middlesex
Hospital Medical School, London W1P 6DB, U.K.
106
J. C. SMITH
removal of the ZPA from a limb does not affect the sequence of digits along the
antero-posterior axis, even though the ZPA is not regenerated (Saunders, 1972;
MacCabe et al. 1973; Tickle et al. 1975; Fallon & Crosby, 1975). This has led
to the suggestion that the ZPA is effective only very early in limb development
and that thereafter its influence can be remembered by the responding cells
(Tickle et al. 1975; Fallon & Crosby, 1975, 1977). In the experiments described
here I support this conclusion by showing that the effect of a grafted ZPA may
also be remembered.
The method takes advantage of the discovery that a graft of a quail ZPA
treated with 10000 rad y-radiation is capable of producing a complete reduplication (Smith et al. 1978). Because the irradiated cells cannot divide
normally the graft tends to remain at the base of the limb, too far from the tip to
exert its polarizing activity; Summerbell (1974a) found that a graft of a ZPA to
a proximal level of a late limb does not produce a reduplication, presumably
because the distance over which it can signal through proximal tissues is limited.
It is shown that the signalling ability of the graft is not passed on to more distal
tissue, and this demonstrates that a reduplication can be obtained without the
continual presence of an additional ZPA.
MATERIALS AND METHODS
Fertilized White Leghorn eggs were incubated at 37-5 °C and windowed on
the third day of development. The embryos were staged according to Hamburger
& Hamilton (1951), sealed with Sellotape, and returned to the incubator. The
eggs were examined at intervals, and those at stage 17/18 were used as hosts.
A graft site was prepared by excising a small piece of tissue, about 200 /tm cubed,
from the right wing bud opposite somite 16.
Quail embryos at stages 21-24 were irradiated as described previously (Smith
et al. 1978) with 10000 rad y-radiation, and pieces of ZPA tissue, also about
200 /on cubed, were transfixed with a platinum pin and positioned in the host
embryos. Camera-lucida drawings of the host limbs were made immediately
after the operation, and approximately 12, 24 and 36 h later. The grafted tissue
could be recognized for at least 24 h after the operation because it appeared
white against the host limb. Only embryos in which the graft could clearly be
seen to be left behind at the base of the limb, and in which the operated limb
bud had widened symmetrically, were used in next stage of the experiment. The
wings of the remainder were fixed and stained by the method below at 10 days
of incubation, and the results are not considered here.
After 36 h each suitable embryo was treated in one of three ways:
1. Embryos allowed to continue development
In the first group embryos were allowed to develop to 10 days of incubation,
and then both wings were fixed in 5 % trichloroacetic acid, stained in 0 1 %
Positional memory in chick wing-bud development
107
Alcian green 2GX in 1 % concentrated hydrochloric acid in 70 % alcohol, differentiated in acid alcohol, dehydrated and cleared in methyl salicylate.
2. Histological examination
In a second group the operated wing bud was fixed in half-strength Karnovsky's fixative (Karnovsky, 1965), dehydrated, and embedded in Araldite.
1£/MTI serial sections were cut in a plane containing the proximo-distal and
antero-posterior axes of the limb, and they were stained alternately with
toluidine blue and by the Feulgen technique. These sections were to confirm that
the grafted tissue had remained at the base of the limb.
3. Polarizing activity assay
In the last group the operated limb bud was removed from the embryo and
placed in a dish of Hanks Balanced Salts Solution. Pieces of tissue from the
anterior and posterior margins were assayed for polarizing activity by grafting
them to the anterior margins of wing buds of host embryos at stages 18-21. In
some cases the tip of the operated bud was allowed to continue its development
by grafting it, in its normal orientation, to the stump of a stage-18 or stage-19
wing bud. These embryos were allowed to develop for a further 6 days before
the limbs were fixed, stained and whole-mounted by the method above. The
unoperated wing bud was fixed, stained and whole-mounted. It was staged by
measuring its proximo-distal length and referring to Summerbell (1976).
RESULTS
Sixty-two grafts of irradiated quail ZPA were made to stage-17/-18 hosts. In
25 of these the ZPA was clearly left at the base of the limb, and the limb had
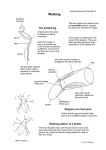
widened symmetrically. A typical series of camera-lucida drawings is shown in
Fig. 1. The widening of the limb is one of the earliest responses to a ZPA graft,
and indicates that a reduplicated limb will be formed. In the 37 other cases
either the grafted tissue was carried forward as the limb grew out, or the limb
bud failed to widen sufficiently, or both. The last camera-lucida drawing was
made about 36 h after the graft, when the embryo was at stage 24 or 24/25, and
the limbs were then treated as described in the Methods.
1. Embryos allowed to continue development
Nine embryos were allowed to continue development, of which seven survived to 10 days of incubation. All produced good reduplications, five with
digit 4 posterior to the graft, and two with digit 3 posterior to the graft (Fig. 2).
2. Histological examination
Three limbs were examined histologically 36 h after the operation to confirm
the position of the graft. The grafted tissue was easily recognizable even in
sections stained with toluidine blue (Fig. 3). The cells were larger than the surrounding host mesoderm cells, and there were macrophages present. The cell
J. C. SMITH
0-3 mm
Fig. 1. Camera-lucida drawings of an operated limb at different times after a graft
of an irradiated quail ZPA. (A) Immediately after the operation. (B) 12 h after the
operation. (C) 24 h after the operation. (D) 36 h after the operation. Notice the
increase in width of the limb. Stippling represents the grafted tissue. The areas a, b,
c, and d represent the tissue assayed for polarizing activity (sometimes only one piece
from the posterior margin was taken). The tip of the limb is also indicated.
density in the graft was lower than in the host, and there was a well-defined
border between the two. The nuclei of the grafted cells stained weakly with
Feulgen, so that the quail nucleolar marker (Le Douarin, 1973) was indistinct.
In all three cases the main body of the graft was positioned at the base of the
limb, and a thin layer of cells extended distally up to ahnost half the length of
109
Positional memory in chick wing-bud development
A "• .
Fig. 2. (A) A normal chick wing. The pattern of digits, beginning at the anterior
margin, is 2 3 4. (B) A reduplicated wing, resulting from a graft of an irradiated
quail ZPA that was left behind at the base of the limb. The digit pattern is4 3 2 3 4.
Fig. 3. A histological section, stained with toluidine blue, to confirm the position of
the grafted tissue. (A) Scale bar is 0-2 mm. (B) The area outlined in A. Scale bar is
0 0 5 mm. The irradiated tissue can be recognized by the large cells, the low cell
density, and by the presence of macrophages (M). Notice that the irradiated tissue
extends distally along the limb, just under the ectoderm.
the limb, just under the ectoderm. These cells could have been dragged by the
ectoderm, which slides distalwards over the mesoderm (Searls & Zwilling, 1964;
Amprino, 1965).
3. Polarizing activity assays
Tissue from 13 operated limbs was assayed for polarizing activity, as shown
in Fig. 1D. Twelve grafts of the more distal piece of tissue on the anterior
margin (piece a, Fig. ID) survived, and none of these grafts produced reduplications in the host wing (Fig. 4A). Quite frequently, however, there were
8
EMB 52
110
J. C. SMITH
abnormalities in the cartilage pattern of the host limb. In two limbs the humerus
was short and thick, and in another two the radius was thick. A further two
limbs had a spur of cartilage coming from the elbow. Twelve grafts of the
proximal piece of tissue on the anterior margin (piece b, Fig. ID) survived. Of
these nine produced no reduplication and three produced an extra digit 2 in the
host limb (Fig. 4B). It is possible that the pieces of tissue grafted in these three
cases contained some of the original graft. This would produce the weak
polarizing activity that was found. As a control, ZPA tissue from the posterior
margin of the limb (pieces c and d, Fig. ID) was grafted. Eighteen operations
survived, and 17 gave good reduplications, 13 with digit 4 (Fig. 4C) and four
with digit 3 posterior to the graft. One gave only an extra digit 2. Finally, six
grafts of the tip of the limb survived. All gave a reduplicated hand on the host
humerus or forearm. Three of these had digit 4 anteriorly, in two cases rather
reduced (Fig. 4D), and three had digit 3 anteriorly. The lack of a digit 4 in these
cases might be because presumptive digit 4 was removed in the assay for
polarizing activity.
It is clear from these results that the signalling ability of the irradiated ZPAs is
not passed on to more distal tissue. Twenty-one out of 24 pieces of tissue from
the anterior margin gave no reduplication, and it seems likely that the polarizing
activity found in three pieces (all of them from next to the grafted tissue) was
due to contamination by the graft. The controls, grafts of ZPA tissue, gave good
reduplications in 17 out of 18 cases.
DISCUSSION
The signalling ability of an irradiated ZPA left behind at the base of a limb as
the limb grows out is not transmitted to more distal tissue. This has enabled me
to demonstrate that the continued presence of an additional ZPA is not required
to obtain a reduplicated limb. I shall discuss these two points separately.
The propagation of the ZPA
Maps of the ZPA (for example, MacCabe et al. 1973) show that its activity is
found progressively more distal as development proceeds. There are two
theories suggesting how this occurs. The earlier is that of Saunders (1972) who
proposed that ZPA cells induce cells distal to them to become ZPA. In contrast,
Summerbell & Tickle (1977) suggested that cells which are to be ZPA are
clonally propagated throughout limb development. The results in this paper
show that the signalling ability of an irradiated ZPA is not transmitted distally,
and this supports the view that ZPA activity is clonally propagated. One
reservation is that irradiation, which attenuates positional signalling (Smith
et al. 1978), might similarly abolish the ability of the ZPA to induce adjacent
tissue to become ZPA (Summerbell, personal communication). However, as I
discuss below, it is clear that distal propagation of ZPA activity is not required
to produce a reduplication.
Positional memory in chick wing-bud development
111
A
1 mm
D
1 mm
Fig. 4. Typical results of the assays for polarizing activity. (A) The result of a graft of
a distal piece of tissue from the anterior margin (piece a). The digits are normal, but
the humerus is malformed. (B) The result of a graft of a proximal piece of tissue on
the anterior margin (piece b). The digit pattern is 2 2 3 4. The radius is missing. (C)
The result of a graft of ZPA tissue from the posterior margin (piece c or d). The digit
pattern is 4 3 2 3 4. (D) A reduplicated hand on a host humerus, resulting from a
graft of a tip onto the stump of a stage-18 embryo. The digit pattern is 4 3 2 3 4, and
and the anterior digit 4 is somewhat reduced.
A positional memory
Grafts of a quail ZPA treated with 10000 rad y-radiation to a stage-17/-18
wing bud produce a complete reduplication even though the graft remains at
the base of the limb and the signalling ability of the ZPA is not transmitted to
adjacent tissue. By stage 24, when the digits have yet to be specified (Summerbell,
19746), the ZPA is too far from the tip to exert its polarizing activity (Summerbell, 1974a). It follows that the cells at the tip remember their exposure to the
ZPA when they go on to form a reduplication. They may be said to have a
positional memory. It is not known how long cells must be exposed to the ZPA,
but preliminary experiments in which an additional ZPA is removed at different
times after the graft suggest that it is between 12 and 17 h (J. C. Smith, work in
progress). The ability of cells to remember their positional value explains why
removal of the ZPA from a limb does not affect pattern along the anteroposterior axis. Such experiments can no longer be interpreted as showing that
the ZPA has no role in limb development.
112
J. C. SMITH
This example of positional memory illustrates the more general point that
positional value is a stable cell state that does not depend upon the continued
presence of any positional cue (Wolpert & Lewis, 1975; Lewis, Slack & Wolpert,
1977). Such stability of positional value is well illustrated in amphibian limb
regeneration, where positional values ascribed during embryonic development
direct the development of the regenerate. A special case of this has recently been
described by Slack & Savage (1978 a, b) who find that amputation of a reduplicated axolotl limb is followed by the regeneration of a reduplicate. Another
example is seen in the cockroach leg, where grafting experiments reveal that
cells in the epidermis are aware of their circumferential position, and apposition
of tissues with different positional values brings about intercalary regeneration
(French, Bryant & Bryant, 1976).
Positional values can, however, change, as in the ZPA grafts performed here
and by many others (see Introduction). It has been suggested by Tickle et ah
(1975) for the chick limb that positional value is stable to a decrease in morphogen concentration but can be raised by an increase in morphogen concentration.
This rule has also been applied to the insect egg by Meinhardt (1977), and may
be useful in elucidating the mechanisms by which positional information is
interpreted (Lewis et al. 1977; Meinhardt, 1978).
I thank Dennis Summerbell, Cheryll Tickle, Fiona Watt and Lewis Wolpert for their
encouragement and comments on the manuscript. I also thank the MRC for a research
studentship.
REFERENCES
R. (1965). Aspects of limb morphogenesis in the chicken. In Organogenesis (ed.
R. L. De Haan & H. Ursprung), pp. 255-281. New York: Holt, Rinehart & Winston.
FALLON, J. F. & CROSBY, G. M. (1975). Normal development of the chick wing following
removal of the polarizing zone. /. exp. Zool. 193, 449-455.
FALLON, J. F. & CROSBY, G. M. (1977). Polarizing zone activity in limb buds of amniotes. In
Vertebrate Limb and Somite Morphogenesis (ed. D. A. Ede, J. R. Hinchliffe & M. Balls),
pp. 55-69. Cambridge and London: Cambridge University Press.
FRENCH, V., BRYANT, P. J. & BRYANT, S. V. (1976). Pattern regulation in epimorphic fields.
Science, N.Y. 193, 969-981.
HAMBURGER, V. & HAMILTON, H. L. (1951). A series of normal stages in the development of
the chick embryo. /. Morph. 88, 49-92.
KARNOVSKY, M. J. (1965). Formaldehyde-glutaraldehyde fixative of high osmolarity for use
in electron microscopy. /. Cell Biol. 27, 137a.
LE DOUARIN, N. (1973). A biological cell labelling technique and its use in experimental
embryology. Devi Biol. 30, 217-222.
LEWIS, J. H., SLACK, J. M. W. & WOLPERT, L. (1977). Thresholds in development. /. tlieor.
Biol. 65, 549-590.
MacCABE, A. B., GASSELING, M. T. & SAUNDERS, J. W. Jr. (1973). Spatiotemporal distribution of mechanisms that control outgrowth and anteroposterior polarization of the limb
bud of the chick embryo. Mech. Ageing Devi 2, 1-12.
MacCABE, J. A. & PARKER, B. W. (1975). The in vitro maintenance of the apical ectodermal
ridge of the chick embryo: an assay for polarizing activity. Devi Biol. 45, 349-357.
MacCABE, J. A. & PARKER, B. W. (1976). Evidence for a gradient of a morphogenetic factor
in the developing chick wing. Devi Biol. 54, 297-303.
AMPRINO,
Positional memory in chick wing-bud development
MEINHARDT,
113
H. (1977). A model of pattern formation in insect embryogenesis. /. CellSci. 23,
117-139.
H. (1978). Space-dependent cell determination under the control of a morphogen
gradient. /. theor. Biol. 74, 307-321.
SAUNDERS, J. W. Jr. (1972). Developmental control of three-dimensional polarity in the avian
limb. Ann. N.Y. Acad. Sci. 193, 29-42.
SAUNDERS, J. W. Jr. (1977). The experimental analysis of chick limb bud development. In
Vertebrate Limb and Somite Morphogenesis (ed. D. A. Ede, J. R. Hinchliffe & M. Balls),
pp. 1—24. Cambridge and London: Cambridge University Press.
SAUNDERS, J. W. Jr. & GASSELING, M. T. (1968). Ectodermal-mesenchymal interactions in
the origin of limb symmetry. In Epithelial-Mesenchymal Interactions (ed. R. Fleischmajer
& R. E. Billingham), pp. 78-97. Baltimore: Williams and Wilkins.
SEARLS, R. L. & ZWILLING, E. (1964). Regeneration of the apical ectodermal ridge of the
chick limb bud. Devi Biol. 9, 38-55.
SLACK, J. M. W. & SAVAGE, S. (1978 a). Regeneration of reduplicated limbs in contravention
of the complete circle rule. Nature, Lond. Ill, 460-461.
SLACK, J. M. W. & SAVAGE, S. (19786). Regeneration of mirror symmetrical limbs in the
axolotl. Cell 14, 1-8.
SMITH, J. C, TICKLE, C. & WOLPERT, L. (1978). Attenuation of positional signalling in the
chick limb by high doses of y-radiation. Nature, Lond. 272, 612-613.
SUMMERBELL, D. (1974tf). Interaction between the proximo-distal and antero-posterior coordinates of positional value during the specification of positional information in the early
development of the chick limb bud. /. Embryol. exp. Morph. 32, 227-237.
SUMMERBELL, D. (19746). A quantitative analysis of the effect of excision of the AER from
the chick limb bud. /. Embryol. exp. Morph. 32, 651-660.
SUMMERBELL, D. (1976). A descriptive study of the rate of elongation and differentation of the
skeleton of the developing chick wing. /. Embryol. exp. Morph. 35, 241-260.
SUMMERBELL, D. & TICKLE, C. (1977). Pattern formation along the antero-posterior axis of
the chick limb bud. In Vertebrate Limb and Somite Morphogenesis (ed. D. A. Ede, J. R.
Hinchliffe & M. Balls), pp. 41-53. Cambridge and London: Cambridge University Press.
TICKLE, C, SUMMBERELL, D. & WOLPERT, L. (1975). Positional signalling and specification of
digits in chick limb morphogenesis. Nature, Lond. 254, 199-202.
WOLPERT, L. (1969). Positional information and the spatial pattern of cellular differentiation.
/. theor. Biol. 25, 1-47.
WOLPERT, L. & LEWIS, J. H. (1975). Towards a theory of development. Fedn Proc. Fedn Am.
Socs exp. Biol 34, 14-20.
WOLPERT, L., LEWIS, J. & SUMMERBELL, D. (1975). Morphogenesis of the vertebrate limb. In
Cell Patterning, Ciba Foundation Symposium 29 (new series), pp. 95—130.
MEINHARDT,
{Received 8 November 1978, revised 25 January 1979)