* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 5-Bioinorganic Chemistry
Western blot wikipedia , lookup
Protein moonlighting wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Biosynthesis wikipedia , lookup
Protein adsorption wikipedia , lookup
Catalytic triad wikipedia , lookup
Biochemistry wikipedia , lookup
Proteolysis wikipedia , lookup
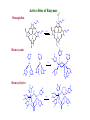
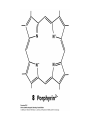
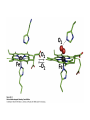
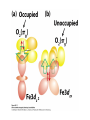
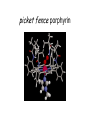
Bioinorganic Chemistry Study of metal species in biological systems •metal ion transport and storage, •Metallohydrolase enzymes, •metal-containing electron transfer proteins, •oxygen transport and activation proteins, •bioorganometallic systems such as hydrogenases and alkyltransferases, •enzymes involved in nitrogen metabolism pathways. Biological functions of selected metal ions Metal Function Na, K Charge carrier, osmotic balance Mg, Zn Structural, hydrolase, isomerase Ca Structural, charge carrier V, Mo Nitrogen fixation, oxidase Mn Photosynthesis, structural, oxidase Fe, Cu Dioxygen transport and storage, electron transfer, oxidase Ni hydrogenase, hydrolase Na Mg K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Y Zr Nb Mo Tc Ru Rh Pd Ag Cd La Hf Ta W Re Os Ir Pt Au Hg Naturally occurring in biology Used as probes Chemical elements essential to life forms can be divided into the following (i) Bulk elements: C, H, N, O, P, S (ii) Macrominerals and ions: Na, K, Mg, Ca, Cl, PO43-, SO42- (iii) Trace elements: Fe, Zn, Cu (iv) Ultratrace elements comprises of (a) non-metals: F, I, Se, Si, As, B (b) metals: Mn, Mo, Co, Cr, V, Ni, Cd, Sn, Pb, Li Essentiality of elements is defined by (1) A physiological deficiency appears when the element is removed from the diet (2) The deficiency is relieved by the addition of that element to the diet (3) A specific biological function is associated with the element Every essential element follows a dose-response curve At lowest dosages organism does not survive In deficiency regions, the organism exists with less than optimal functions After optimal dosage (plateau region), higher dosage cause toxic effects in the organism eventually leading to lethality Active Site and Enzyme Substrate Complex The active site of an enzyme is the region that binds the substrate and contributes the amino acid residues that directly participates in the making and breaking of chemical bonds Generalizations 1) Enzymes are usually very large compared to the substrate Only a small portion is involved in ES complex Rest portion is involved in control and maintaining of structure 2) The substrate is bound by relatively weak forces ΔG E-S complex = (12 to 36) KJ mol-1 (strength of a covalent bond is upto ~ 450 KJ mol-1) 3) Active sites are designed to exclude H2O Surrounded with non-polar amino acids to create a hydrophobic environment Essential for substrate binding and product formation (Catalysis) Specificity Active site provides specificity for its particular substrate Substrate has a matching shape to fit into the active site (Lock and Key mechanism) Formation of Enzyme-Substrate Complex is thus crucial to the product formation Evidence for Enzyme-Substrate Complex (1) At constant [E], increasing the [S] will increase the reaction rate until a maximum velocity is reached, (2) Isolation of E-S complex (3) X-ray diffraction studies of E-S complex (4) Spectroscopic studies of E-S complex Active sites of Enzymes His(N) Zn Glu(O) OH 2 peptide hydrolysis (removes terminal amino acids f rom proteins) His(N) Carboxy peptidase His(N) His (N) Zn OH2 H 2O + CO H 2CO 3 H + + HCO 3- His(N) Carboxy anhydrase NAD+ Cys(S) Cys(S) His(N) Zn NADH OH2 CH 3CH 2OH Liver Alcohol dehydrogenase CH 3CHO Active-Sites of Enzymes O Hemoglobin O CO 2- CO 2N N II Fe N CO 2- O2 N N CO 2- II Fe N N N Hemocyanin NH HN NH N H N N N N HN N H CuII O N N N NH NH O CuII N N N N O2 CuI CuI Nh N N H N H N H Hemerythyrin H N H N H H O N HN O O Fe O HN N H O O FeII N N N O HN O2 II Fe N N HN N II N NH O O O N FeIII N O O N H NH Porphyrins Porphyrins are tetrapyrrole macrocycles with conjugated double bonds and various groups attached to the perimeter R R N R R HN NH R R N R R variation of substituents facilitates the tuning of electron-donating and electron-withdrawing ability of the ligand The porphyrins can accept two hydrogen ions to form+2 diacids or donate two protons to form -2 dianions Porphyrins are found in many metalloenzyme Enzyme Function Fe-porphyrin Cytochrome electron transfer Fe-porphyrin Hemoglobin Myoglobin dioxygen carrier Mg-porphyrin Chlorophyll photosynthesis Cytochromes Cytochromes are electron transfer proteins There are three types of cytochromes depending upon the porphyrin types cytochrome a, cytochrome b, cytochrome c s-cys protein s-cys protein HO HO N N N N N N Fe Fe Fe N N N N N N O H HO OH O O a HO OH O O b HO OH O O c The prosthetic group in all cytochromes comprises of four heme units They have a molecular weight of about 12,400 Active site differences between Hemoglobin and Cytochrome O S (cys) O N N N N N Fe Fe N N N N (His) N (His) Hemoglobin Cytochromes Depending upon the ligand, the redox potential of a given cytochrome can be tailored to meet specific need in electron transfer schemes (photosynthetic versus respiration) The potentials are such that the electron flow is from b c a O2 Cytochrome a is capable of binding O2 and reducing them Cytochrome a is responsible for severe toxicity of CNCN- binds to the 6th site and stabilize FeIII to such an extent that it cannot participate in electron transfer schuttle Hemoglobin O O N N N N Fe II N N Fe II N N N (His) N (His) High Spin paramagnetic t2g4 eg2 Deoxyhemoglobin Low Spin diamagnetic t2g6 eg0 Oxyhemoglobin Deoxyhemoglobin is the form of hemoglobin without the bound oxygen. The oxyhemoglobin has significantly lower absorption (660 nm) than deoxyhemoglobin (940 nm). This difference is used for measurement of the amount of oxygen in patient's blood by pulse oximeter. The size of Fe2+ increase by 28% on going from Low spin (oxyhemoglobin) (0.61 Å) to High spin (Deoxyhemoglobin) (0.78 Å) The Fe2+ in deoxyhemoglobin is too large to fit in the ring and is situated (0.7-0.8)Ao above the ring Thus, presence of O2 changes the electronic arrangement of Fe2+ and distorts the shape of the complex The globular protein prevents the irreversible oxidation of Fe(II) to Fe(III) Cooperativity When O2 binds to one sub-unit Fe2+ contracts, moves into plane of porphyrin ring triggers conformational changes in the globin chain moves the histidine attached to it translated through H-bond network Enhances the ability of other three units to bind O2 This phenomenon is called cooperative effect In a similar way when the blood reaches the muscle, only one O2 is released, the others are released even more easily due to the cooperative effect in reverse picket fence porphyrin