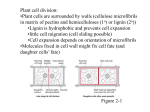
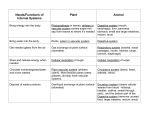
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Physiological correlates of the morphology of early vascular plants
History of phycology wikipedia , lookup
Indigenous horticulture wikipedia , lookup
Plant tolerance to herbivory wikipedia , lookup
History of herbalism wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Plant stress measurement wikipedia , lookup
Venus flytrap wikipedia , lookup
Cultivated plant taxonomy wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Ornamental bulbous plant wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Historia Plantarum (Theophrastus) wikipedia , lookup
History of botany wikipedia , lookup
Photosynthesis wikipedia , lookup
Flowering plant wikipedia , lookup
Plant evolutionary developmental biology wikipedia , lookup
Plant morphology wikipedia , lookup
Plant physiology wikipedia , lookup
Sustainable landscaping wikipedia , lookup
Botanical Journal of The Linncan So&& (1984),88: 105-126 Physiological correlates of the morphology of early vascular plants JOHN A. RAVEN Department of Biological Sciences, University of Dundee, Dundee DDl 4HN Received January 1983, acceptedfor publication A U ~ U 1983 S~ RAVEN, J. A,, 1984.Physiological correlates of the morphology of early vascular plants. The early evolution of vascular land plants is considered in relation to the physiological problems of life on land. The universal characteristics of vascular plants (xylem, cuticle, stomata, intercellular air spaces, long-distance symplastic transport and alternation of generations) are discussed in terms of the essential properties of a homoiohydric phototroph. Likely precursors of vascular plants, and the physico-chemical and biotic environment in which they occurred, are outlined prior to a discussion of the selective forces acting on the evolution of vascular plants in the Upper Silurian and Lower Devonian. Emphasis is placed on biochemical and structural ‘pre-adaptations’ which may have occurred in the precursors of vascular plants and on which natural selection could have acted with lignified xylem, stomata, etc., as the end-products. Guiding principles in the analysis include the physiology of extant plants, physico-chemical constraints, and compatibility with the fossil record. I t is concluded that the likely sequence of acquisition of vascular plant characteristics was: heteromorphic alternation of generations with an erect sporophyte; cuticularization of sporophyte; evolution of xylem; occurrence of intercellular air spaces with pores in the epidermis; stomata1 activity of the pores. Endodermis and phloem-type long-distance transport probably originated around stages (3)-(5). KEY WORDS:-Cuticles - Devonian - endodermis - homoiohydry - intercellular air spaces lignin - non-vascular land plants - phloem - Silurian - stomata - vascular land plants - xylem. CONTENTS Introduction . . . . . . . . . . . . . . . . . . . The vascular condition. . . . . . . . . . . . . . . . . The physico-chemical and biotic environment of the early evolution of vascular plants . How vascular plants may have arisen . . . . . . . . . . . . . Conclusions . . . . . . . . . . . . . . . . . . . Acknowledgements . . . . . . . . . . . . . . . . . Appendix 1: T h e relative specific H,O conductance of parenchyma and xylem tissues . Appendix 2: T h e H,O loss associated with photosynthetic CO, fixation in a terrestrial plant . . . . . . . . . . . . . . . . . Appendix 3: The significance of exposing large areas of cell surface to the gas phase in relation to the plant’s investment in RUBISCO and in structure . . . Appendix 4: The significance of xylem water transport for supplying water to cells . . . . . . . photosynthesizing with air as the CO, source. Appendix 5: T h e costs of homoiohydry and desiccation-resistance . . . . . . References . . . . . . . . . . . . . . . . . . . 0024-4074/84/020105 +22 803.00/0 106 106 I10 111 115 116 I16 I16 117 I19 120 122 I05 0 1984 The Linnean Society of London 106 J. A. RAVEN INTRODUCTION Kenneth Sporne kept British plant morphology alive as a discipline at the undergraduate and postgraduate level while all around there were defections to palaeobotany per se, to electron microscopy, and to developmental biology. His research and teachings concentrated on the contribution which classical anatomy and morphology had to make to our understanding of how plants were related to each other, and he was always scrupulous in distinguishing fact from speculation. While trying to keep to this latter precept, my facts and speculations will be drawn from physiology to at least as great an extent as from morphology and anatomy. My aim is to comment on some of the possible selective pressures, and the processes, involved in the evolution of a vascular land flora, to think about the events and processes at the very beginning of the story of pteridophytes, gymnosperms and angiosperms which is so clearly told in the three textbooks of vascular plant morphology produced by Sporne over the last two decades (Sporne, 1974a,b, 1975). The present essay is essentially a precis, extension and reformulation of Raven (1977a), with contributions from: Raven (1970, 1977b, 1980, 1981a, b, 1983a, b), Raven & F. A. Smith (1976), Raven, F. A. Smith & S. E. Smith (1980) and Raven, S. E. Smith & F. A. Smith (1978) and many others acknowledged in this article. I have tried to heed warnings from Garland ( 1981 ) as to the difficulties in applying Occam’s razor to evolutionary speculation, and of Osmond, Bjorkman & Anderson (1980) as to the difficulties in attributing selective significance to physiological attributes even in extant plants. THE VASCULAR CONDITION In order to put the evolution of vascular plants into perspective it is necessary to consider the essential attributes of a vascular plant sporophyte, and their significance. The vascular land plant lives at the interface between a liquid phase (the soil solution, supplying H,O and nutrients, e.g. N, P and K, other than CO,, with the solid phase of the soil providing support, and generally a gas phase present in the soil as well) and the atmospheric gas phase (supplying CO, and transparent to photosynthetically active radiation (400-700 nm)). There are substantial differences between this pattern of resource availability and that of a submerged benthic rhizophyte (cf. Luther, 1949; Raven, 1981b) in that H,O is available to the terrestrial vascular plant only via the rooted portion and, indeed, is lost from the shoot system as a concomitant of CO, fixation. An even larger contrast is found in comparison with benthic submerged haptophytes (and phytoplankters) where CO,, other nutrients and water, as well as light, can be absorbed all over the plant surface. A major attribute of the vascular land plant is the homoiohydric condition (see Raven, 1977a; Walter & Stadelmann, 1968); the mechanism and significance of homoiohydry is a major theme of this article. Extant terrestrial vascular plants are characterized by the possession of: xylem; cuticle; stomata; intercellular air spaces; phloem; endodermis (or its functional equivalent); alternation of generations. Xylem: This is the sine qua non of vascular plants, and is essentially a means of transmitting large quantities of H,O up the plant under relatively low H,O PHYSIOLOGY AND FOSSIL PLANTS I07 potential (#w) gradients (Appendix 1). A large water flux from soil to shoot is needed to supply not only the 5-10 kg H,O retained in shoot material for every kg dry weight increment in shoot growth, but also the 300 or more kg of H,O transpired per kg dry weight gain as a concomitant of CO, fixation (Appendix 2). Since the xylem H,O is under tension (Appendix 1; Pickard, 1981) there is a tendency for the xylem elements to collapse, the tension in the contained water being perceived as a compressive force in the wall. This tendency to collapse is resisted by the presence of lignin (complex polymers of phenylpropanoid units: Gross, 1980). The low resistance to longitudinal (axial) water flow in the xylem is related to the absence of living cell contents: programmed cell death as a part of the development of phototrophic organisms at many phylogenetic levels, for example, the ‘necridia’ of cyanobacteria (Addicott, 1982; Lamont, 1969). Lignin also functions in anti-biotroph defences (probably preceding its structural role in xylem in compression resistance in H,O-conducting elements) and in the support of the plant shoot in the low-density fluid medium of air (probably a later role than its function in conducting elements): Raven (1977a). Cuticle: The cuticle is a hydrophobic, water-repellent and water-impermeable layer over the surface of the shoot and; indeed, at all cell-air interfaces such as on root surfaces, and the linings of intercellular gas spaces (Hadley, 1981; Raven, 1977a). The cuticle per se consists of a complex mixture of cross-linked long-chain esters, alcohols and fatty acids, and has a wax (long-chain hydrocarbon) layer on its surface. The water permeability is not readily related to the cuticle thickness (Martin &Juniper, 1970; Schonherr & Merida, 1980); it is largely controlled by the composition and thickness of the wax layer. For a given number of wax molecules per unit shoot surface area the H,O permeability is approximately halved for each additonal two-carbon unit added to the hydrocarbons (paraffins) in the wax, thus rationalizing the occurrence of such long-chain hydrocarbons in the wax (Schonherr & Merida, 1980). This outer wax layer facilitates H,O run-off following rain or dew, thus decreasing the resistance to CO, influx; it also forms a layer which, when complete (i.e. when stomata are closed or absent) very greatly reduces the H,O permeability of the plant surface, thus reducing water loss from a plant whose H,O supply cannot keep pace with the evaporative demand of the atmosphere but also, alas, reducing CO, entry and photosynthetic fixation, since no known cuticle-wax complexes have a significantly higher permeability to CO, or 0, than to H,O (see Lendzian, 1982; Raven, 1977a). The negligible resistance to H,O evaporation from the (waxless) walls of mesophyll cells lining intercellular spaces in the leaf does not increase with decreased water content in contrast to the situation in many poikilohydric plants (Jones & Higgs, 1980; Jones & Norton, 1979; Proctor, 1979). The cuticle (plus wax) also acts as a defence against biotrophs, and, in plants growing in environments with a high photon flux density, thick cuticles can reflect deleterious radiation of both UV and longer wavelengths Klein ( 1978). Sfornufa: The variable-aperture stomatal pores are in the epidermis and cuticle; when open, they permit gas exchange between the external atmosphere and the internal gas phase of the shoot (see Appendix 1); when closed, gas exchange is greatly restricted. The regulation of stomatal conductance appears to operate to optimize CO, gain in photosynthesis per unit H,O lost in transpiration I08 J . A. RAVEN (Cowan, 1977; Wong, Cowan & Farquhar, 1979; see Jones, 1976, for a discussion of the effect of different fractions of leaf photosynthesis being consumed in dark respiration on this optimization). Internal air spaces: These are the necessary correlate of stomata. Without an internal aeration system to ‘distribute’ CO, in a medium in which CO, diffuses lo‘ times faster than it does in H,O, the photosynthetic rate per unit plant external surface would be much lower than the rate achieved by homoiohydric plants. Appendix 3 discusses how the balance between diffusive and biochemical constraints on the rate of CO, fixation is struck. In brief, the large N and energy input in producing the primary photosynthetic carboxylase, ribulose bisphosphate carboxylase-oxygenase (RUBISCO), is not optimally used (in terms of CO, fixed s/unit N or unit energy invested in producing the enzyme) unless the enzyme is displayed over a large area of cell-gas phase interface with a minimal aqueous-phase diffusion path. Most terrestrial vascular plants have about one-third of their total limitation on photosynthesis in transport (diffusion) processes in the gas phase of the boundary layer, stomata and intercellular air spaces, and the liquid phase; the remaining two-thirds of the resistance is attributable to biochemical processes including RUBISCO activity (Begg, 1980; Caemmerer & Farquhar, 1981; Nobel, 1980; O’Leary, 1981; Raven, 1981a). Thus the intercellular space system is crucial to the gas exchange of the shoots of homoiohydric vascular plants. We may contrast the contention of Church (1919) who asserted that the stomata air space system was not essential for photosynthetic CO, uptake since CO, could cross a wet membrane as easily as a comparable air barrier: this view is clearly wrong and the importance of stomata had been pointed out by Brown & Escombe (1900). Nutrient solute acquisition and transport: Several features not completely restricted to vascular plants are associated with the acquisition of nutrients. For N, P, K and some micronutrients the analyses of Grubb (1977), Nye & Tinker (1977), Oliver & Barber (1966a,b) and Prenzel (1979) show that the soil solution concentration in non-agricultural soils is too low for the H,O flux toward the plant during transpiration to supply the nutrient to the root surface at a rate fast enough to supply the plant’s requirements. In quantitative terms, the P per unit dry weight of plant divided by the H,O transpired per unit dry weight increment of plant exceeds by up to a thousand fold the P concentration in the soil solution, with analagous relations holding for N and K. The supply of these nutrients to the plant is thus a function of the (very slow) diffusion of phosphate (and the much faster diffusion of K + and of the NH: and NO2 which demands that the plant must continually extend its subterranean portions into relatively unexploited parts of the soil in order to obtain sufficient of these elements (Nye & Tinker, 1977; Scott-Russell, 1977). In extant vascular plants these extending organs are roots and/or rhizomes bearing root hairs (rhizoids) and/or mycorrhizas (see Smith, 1980). The extension rate through soil is, of course, limited by the need to concentrate growth in a limited apical region otherwise buckling of the extending organ would occur in the mechanically resistant soil (see Scott-Russell, 1977). It is likely that, in well-watered soil, the extent of the root system is a function of nutrient acquisition capacity rather than the need for mechanical support for the shoot. The plant’s nutrient requirements demand selective uptake by the plant; PHYSIOLOGY AND FOSSIL PLANI'S 109 further, selective transfer to the shoot is required in view of the very limited capacity for the shoot to dispose of excess solutes by direct excretion or by phloem retranslocation to below-ground portions: particular difficulties are experienced with H + / O H - and C a 2 + (Raven, 1977a,b). Church (1919; see Mabberley, 1981) pointed out the significance of the transpiration stream as the main mode of nutrient transport to the shoot; clearly diffusive transport of solutes within the plant is inadequate over distances of the order of the height of even small vascular plants, since Nobel (1974) showed that the time taken for a solute of diffusion coefficient lo-' m2 s-' to reach I/e (0.37) of its source concentration is 0.6 s for a sink 50 pm distant but 8 years when the sink is 1 m distant from the source! The selectivity of transport into the phloem is maintained by a restriction on apoplastic (extracellular) H , O flux, which could (depending on the properties of the apoplastic pathway) give a non-specific solute entry to the xylem, to some 1-3% of the total H,O flux (Pitman, 1977; Raven, 1983a). The symplastic movement of solutes and H,O permits a much greater selectivity of solute loading into the xylem. The endodermis contains suberin in the casparian strip as an apoplastic barrier; this structure may have originated from the internal cuticle (see Krassilov, 1981), and may also function in maintaining the polarity of solute porters between the stelar and the cortical symplast plasmalemma (see Dragsten, Blumenthal & Handler, 1981, for the analogous role ofanimal epithelial tight junctions). The xylem is apoplastic, and thus does not suffer from the deficiencies of the symplast phloem with respect to the carriage of such solutes as H + and C a 2 + (Raven, 1977a,b). However, the H , O carried by the xylem ends u p mainly in mature photosynthetic organs which are the main sites of transpiration, rather than in the growing portions of the shoot where there is the major net requirement for the xylem-borne solutes. Pate (1980) and Pate & Layzell (1981) point out that xylem-to-xylem transfer of solutes takes place within the stem, with a depletion of xylem streams destined for the mature photosynthetic organs and an enrichment of those streams passing to immature regions of the shoot in xylem-borne solutes. This transfer, and xylem-to-phloem solute transfer, recalls some older pre-occupations of plant morphologists with stelar morphology in relation to exchanges between the stele and extra-stelar tissues (e.g. Bower, 1930). However, the modern thrust is aimed more at exchanges between stelar elements themselves, and the morphological expression of the surface/volume relationships discussed at length by Bower (1930) is now thought to reside at the level of vascular transfer cells (see Gunning, Pate & Green, 1970) as well as in grosser aspects of vascular structure. As with many transfer cell locations it is not easy to see a taxonomic pattern behind the presence or absence of vascular transfer cells; but it may be significant that xylem transfer cells are lacking in extant lycopsids which lack leaf gaps (Gunning, Pate & Green, 1970; cf. Sporne, 1974a,b, 1975). The symplastic phloem appears to be optimized for the transport of organic C and N with minimum energy expenditure in propelling the phloem sap containing the C and N (Lang, 1977; Passioura, 1976) from C sources to C sinks. As a 'general carrier' the phloem suffers, as does the xylem, from the problem of directionality, since the phloem moves solutes only from photosynthetic or storage regions to growing regions; there is the further 110 J. A. RAVEN problem that certain solutes (H’, C a 2 + )are not transported readily in phloem, at least in comparison to the potential physiological need for their transport. This latter difficulty is quantified by Raven (1977b) via the ratio of elements in the phloem and the ratio of elements required in the sink tissue. Reproduction: All vascular plants show a heteromorphic alternation of generations (Keddy, 1981), and the properties of the homoiohydric plant discussed above relate essentially to the sporophyte generation; the gametophyte generation is essentially poikilohydric, with protection from H,O stress afforded by the megaspore wall of heterosporous free-sporing plants, and by seeds of spermatophytes where the megasporangium is indehiscent. The wind dispersal of spores (and later seeds) was probably a major early selective pressure for plants of greater sporophyte stature, thus allowing the release of propagules into turbulent air. Other advantages relate to the competition for light (see Givnish, 1982) and the avoidance of grazers and parasites which, in the case of the insects and fungi, soon evolved wings and aerially dispersed spores respectively! (see Raven, 1977a; Swain, 1978; Swain & Cooper-Driver, 1981). Despite biophage ‘short-circuiting’ of some of the advantages of size, a large size is still an important adaptive stratagem in extant plants of stable habitats which have reasonable resource availability (the C strategists of Grime, 1979). Such increased size demands quantitative and qualitative additions to the amount and efficiency of the homoiohydric H,O regulation system. THE PHYSICO-CHEMICAL AND BIOTIC ENVIRONMENT OF THE EARLY EVOLUTION OF VASCULAR PLANTS The physico-chemical environment: The description of the physicochemical environment of the Silurian and Devonian presents difficulties. The solar radiation available to the earth may have increased by some 30% over the last 4.109 years (Newman, 1980), although the ultraviolet component may have been much more important early in this period (Canuto et al., 1982); thus photosynthetically active radiation in the Silurian-Devonian was probably similar to extant levels. The CO, content of the atmosphere early on in the earth’s evolution may well have been substantially higher than present-day levels. The biological and geochemical factors regulating this content are discussed by Holland (1965) and Lovelock & Whitfield (1982), while substantial variations over periods of 10’- 1O5 years are documented by Neftel et al. ( 1982). The 0, content of the atmosphere was probably less than the current level, with a recent estimate of some 13 kPa (cf. the current 21 kPa) for the Lower Carboniferous 0, partial pressure probably representing an upper limit for the Siluro-Devonian (Cope & Chaloner, 1980, 1981; cf. Clark & Russell, 1981). Mechanisms regulating atmospheric 0, levels are imperfectly understood (Margulis, 1981 ; Redfield, 1958). The earliest sites for vascular macrofossils were at low latitudes, although it is not clear what the ambient temperatures would have been. The soil would have been very substantially modified by the pre-vascular plant biota discussed below. The biolic environment; It is important to realize that there was probably a substantial non-vascular phototrophic flora on land before the first vascular land plants. This consisted of ‘soil algae’, probably mainly members of the PHYSIOLOGY AND FOSSIL PLANTS Ill Chlorophyta and Cyanobacteria (Metting, 1981) as well as the metaphyta which possessed trilete spores and thick cuticles (Chaloner & Sheerin, 1979). Such organisms could have been involved in pedogenesis, including the release of mineral nutrients (K, P) into solution and the fixation of N, to give ‘combined N’ (see Gorham, Vitousek & Reiners, 1979). Furthermore, there was probably a substantial range of heterotrophs present, including fungi and arthropods. Some were presumably necrophages, but others were probably biophages, and the production of secondary products by the phototrophs was probably an early counter-measure (see Raven, 1983b). Extant soil and freshwater Chlorophyta produce phenolics and sporopollenin (Raven, 1977a); these compounds could have had a role in mitigating the effects of UV light which could have been more significant when the oxygen/ozone screen was less complete (see Margulis, 1981). Cuticle-like structures can reflect UV light, and epidermal phenolics (Lowry, Lee & Hebant, 1980) can absorb UV light before it has penetrated to ‘sensitive’ DNA, RNA and proteins. These compounds are important precursors of essential elements of the homoiohydric apparatus of vascular plants; Gomes & Gottlieb (1978) make a contrary suggestion (based on little evidence) that chemoprotectant micromolecules are secondary to the macromolecular phenolic polymer lignin which had (and has) a mechanically protective role in preventing biophagy. HOW VASCULAR PLANTS MAY HAVE ARISEN How large were the transmigrant algae?: The fossil record is not very helpful in indicating the origin of vascular plants. Biochemical and ultrastructural studies of extant plants suggest that it is the algal division Chlorophyta, and more specifically the class Charophyceae, which is most closely related to the Bryophyta and Tracheophyta (Graham, 1982; Graham & Wilcox, 1981; Melkonian, 1982; Raven, 1977a; Stebbins & Hill, 1980; Stewart & Mattox, 1978; Taylor, 1982; Whatley, 1982). The general (ultrastructural, enzymic) attributes of the Charophyceae are not readily ascertained from fossils, so only more specific attributes which associate a fossil with an extant charophycean alga can indicate their occurrence. Upper Silurian gyrogonites (oogonia), and Lower Devonian rhizoids, suggest that the specialized charophycean macroalgae were contemporaries of the earliest vascular plants, but give little information on the nature of the presumed common ancestor of the Charophyceae and the Tracheophyta (Edwards & Lyon, 1983; Grambast, 1974). Various hypotheses have been put forward as the degree of morphological complexity of the transmigrant alga. Church ( 1919) suggested transmigration from an aquatic environment of a large, complex plant, while Stebbins & Hill ( 1980) adopted an antithetic stance in proposing unicellular tranmigrants; authors such as Bower (1930), Fritsch (1945) and Raven (1977a) occupy the middle ground. Church (1919) maintained that vascular plants derived from ‘stranded’ benthic haptophytic marine algae; such algae had the biochemical attributes of green (we would now say charophycean) algae, but a parenchymatous thallus rivalling in complexity that of extant Laminariales or Fucales in the I12 J. A. RAVEN Phaeophyceae, together with fertilization in situ and an alternation of generations. These ‘Thalassiophyta’ no longer exist (if they ever did). In contrast, Stebbins & Hill (1980) suggested that members of the Charophyceae are essentially soil-dwelling green algae, and that the evolution of structural complexity led to both the predominantly terrestrial Bryophyta and Tracheophyta, and to a multicellular algal residuum, most of which are freshwater submerged aquatics. The supporters of the intermediate view seek the origin of vascular land plants from reasonably differentiated Fritschiella-like benthic freshwater rhizophytes, which became soil-dwellers at a stage of morphological and reproductive complexity intermediate between the unicells of Stebbins & Hill (1980) and the complex parenchymatous (Graham, 1982) plants of Church ( 1919; Corner, 1964). Space does not permit an exhaustive analysis of these different possibilities. However, the views of Stebbins & Hill (1980) and of Fritsch (1945) seem plausible in that the sorts of selective pressure which led to differentiated, perennial multicellular thalli in several classes of marine algae could also operate in freshwater and on land. Such selective pressures include greater immunity from biophages, the ability to shade out competitors at their own trophic level; the ability to sequester seasonally available nutrients; and the ability to place propagules into turbulent, fast-flowing fluid (H,O or air): see Grime (1979); Raven (1977a); Raven (1981b); Raven, Smith & Glidewell (1979); Swain (1977); Swain & Cooper-Driver (1981). Accordingly, characteristics such as multicellularity, well-defined growing zones, plasmodesmata and oogamy could have been selected for in freshwater or on soil just as occurred in the marine Phaeophyceae and Rhodophyceae. Certainly some of the arguments of Church (1919) against a freshwater or soil origin of vascular plants have been falsified by later investigations; examples are the claims of a very marked contrast in N and P availability between seawater and freshwater (Morris, 1980; Moss, 1980), and the inability of marine algal propagules to invade freshwater and terrestrial habitats in the absence of animals to move them. Characteristics of prevascular land plants: A wide range of plants occurred on land in the Silurian; multicellular plants with a cuticle, and spores with sporopollenin, were established by mid-Silurian times (Banks, 1975; Chaloner, 1970; Chaloner & Sheerin, 1978; Edwards, 1979; Edwards, Bassett & Rogerson, 1979; Edwards, Edwards & Rayner, 1982; Edwards, Feehan & Smith, 1983). In addition to the UV-reflective and anti-biophage roles (see above), the very thick cuticles of some of these plants may have had a structural role, as well as being something of an impediment to photosynthetic CO, uptake. However, as noted above, the thickness of the cuticle is not a good guide to H,O permeability. Certainly, a thick, hydrated cuticle might inhibit CO, entry more than the entry of this gas would be facilitated by a non-wettable plant surface increasing ‘run-off of liquid H,O, thus decreasing the liquid phase resistance to CO, transport. The exact nature of the sporangia from which the early and mid-Silurian spores were produced is unknown. An early Silurian fossil which Raven (1977a) took as a model for the immediate precursor of a vascular plant is Eohostimellu: the coalified material PHYSIOLOGY AND FOSSIL PLANTS I13 represents (presumably) erect cylindrical axes 1-2 mm in diameter (Schopf el a f . , 1966); no reproductive structures or details of internal structure are known, although the nature of the preservation is presumed by Schopf el a f . (1966) to indicate the existence of a cuticle and of thick-walled hypodermal tissue (Edwards, Bassett & Rogerson, 1979). Appendix 4 shows how large a difference in H , O potential between the top and bottom of the plant would be needed to supply water at a rate commensurate with a CO, fixation rate of lpmol m - 2 s-I for a plant lacking intercellular air spaces; such a low @ ,,, at the apices would presumably have an adverse effect on the biochemical reactions of photosynthesis (Hsaio, 1973; Larcher, 1979). The evolution of earh vascular plants: The ‘upward urge’ (for the selective reasons discussed earlier) could be sustained only by an improved water supply to the aerial axes. Such an improved supply could be envisioned for Cooksonia, one of the earliest vascular plants so far described. This plant had dichotomizing, cuticularized axes bearing terminal sporangia, and had peripheral (collenchymatous) supporting tissue similar to that presumed by Schopf et a f . (1966) to occur in Eohostimeffa; it also had a central strand of tracheids (Edwards et a f . , 1979 and Edwards el a f . , 1983). The advantage to the plant in terms of H,O supply to the aerial, photosynthetic axes of the presence of xylem is discussed in Appendices 1 and 2. The programmed cell death inherent in xylem production has precedents in prokaryotes (the necridia of cyanobacteria) and in eukaryotic algae (death of some products of meiosis in oogenesis in some fucalean algae: Bold & Wynne, 1977), but is not commonly a part of morphogenesis of the vegetative plant body (see Wetherbee & Kraft, 1981). Lignin (claimed to be present in early Silurian strata containing no vascular plants: Niklas & Pratt, 1980) had precursors in the UV-screening for antibiophage phenolics (see above). The final stage in the evolution of the homoiohydric plant involved the acquisition of the stomata/intercellular air space system. These attributes are both found in Rhynia, but the occurrence of intercellular gas spaces in <osterophyffum (the earliest plant with stomata) is still conjectural (see below). The selective advantage of the intercellular air space system can be readily seen in terms of the improved surface/volume ratio which minimizes the liquid-phase diffusion path of CO, from the gas phase to RUBISCO in organisms which, by growing taller, must for mechanical reasons increase the diameter of their axes (see Niklas, 1978) and thus have a decreased ratio of external surface to volume (see Appendix 4). Other methods of increasing the surface to volume ratio are seen in extant poikilohydric plants such as members of the Polytrichaceae (see Proctor, 1979; Raven, 1977a), but these surface corrugations have less potential than the development of intercellular gas space for the subsequent evolution of homoiohydry. The schizogenous production of gas spaces in an initially (in the meristem) ‘solid’ aqueous tissue is clearly a well-ordered process (Roland, 1978), but the physical details are still poorly understood (see Raven, 1977a). Precedents for the production of intercellular air spaces in parenchymatous tissues seem to be lacking in algae, but an analagous phenomenon is found in the gametophytes of the Marchantiales and in sporophytes of Musci (see Raven, 1977a; Appendix 4). It is possible that intercellular air spaces in the sporophytes of vascular plants were first found in the sporangia, with haploid spores 1 I4 J . A. RAVEN immediately prior to their release being separated from the diploid sporangium wall by an air space; the extent to which this situation is relevant to the production of intercellular spaces in a mass of diploid vegetative tissue is not clear. Stomata may have originated from ‘passive’ aerating pores of the type found in the Marchantiales (Raven, 1977a); their origin as secretors of liquid H,O (Church, 1919) implies that root pressure was occurring and that an endodermis (or its functional equivalent) was present. The acquisition of the ‘active’ opening and closing movements involves the special mechanical properties of the guard cell walls, and the coupling of osmotica-generating and osmotica-removing reactions, which alter cell turgor and thus stomata1 aperture, in response to environmental stimuli of light, intercellular space CO, concentration, relative humidity, leaf H,O potential, abscisic acid concentration and, most importantly, the metabolic capacity for photosynthesis of the mesophyll cells (see Wong, Cowan & Farquhar, 1979). While ~osterophyllumand subsequent vascular plants had (with the proviso of adequate preservation) stomata (see Chaloner, 1970; Chaloner & Sheerin, 1979), it is less easy to be sure that they had intercellular air spaces; this point seems proven for Rhyniu, but not for ,&lerophyllum. It would be most interesting (and puzzling!) if stomata occurred in the absence of intercellular gas spaces. Turning to the quantitative aspect, the frequency of stomata (number mm -,) in the early vascular plants seems to have been lower than the range in extant plants (cf. Stubblefield & Banks, 1978, with Table 1 of Meidner & Mansfield, 1968); however, the dimensions for the stomata of Rhyniu and <osterophyllum stomata (Kidston & Lang, 1917; Walton, 1964) are at the upper end of the range quoted in Table 1 of Meidner & Mansfield (1968); thus the conductance (computed from frequency and dimensions according to Meidner & Mansfield, 1968: 59, equation (6)) is probably a t the low end of the range for extant vascular plants (Woodhouse & Nobel, 1982). This sets limits on the photosynthetic capacity of the plants relative to that of extant plants, although this may have been offset in part by higher ambient CO, concentrations (see above) in the Devonian (cf. Appendix 4). The foregoing arguments suggest that the sequence of acquisition of H,Oconducting and gas-exchange-regulation mechanisms revealed by the fossil record is generally in accord with teleological expectation, in that the H,Oconducting system in the absence of the gas-exchange-regulation mechanism would be of more selective advantage than the reverse state of affairs in an erect plant. Much less is known about the evolution of the below-ground portions of the sporophytes of vascular plants. The diageotropic subterranean axes of Rhyniu and other plants in the assemblage had root hairs and mycorrhizas (inferred from the presence of fungal cysts). The ‘root’ and ‘-rhiza’ terminology is not entirely appropriate with respect to the Rhynie plants which did not have true roots. The ‘root hairs’ and ‘mycorrhizas’, together with the (presumably) apical growth of the axis and its branches, probably served to exploit the nutrient and H,O reserves of the soil. These subterranean organs are assumed to have had an important role in relation to acid-base balance during nitrogen assimilation (Raven & Smith, 1976); Raven et al. (1978) have suggested that the mycorrhizas of these plants may have had a particular role in acid-base PHYSIOLOGY AND FOSSIL PLANTS 115 regulation. In terms of the wider problem of the regulation of solute transfer to the shoot in the transpiration stream it has been argued above that some apoplastic barrier to solute transfer from the soil solution to the xylem is a prerequisite for the regulation of solute supply to the shoot. A well-defined endodermis in fossil vascular plants was apparently a relatively late acquisition (e.g. in Carboniferous Equisetites: Walton, 1940). Before the absence of a welldefined endodermis in the axes of Rhynia (see Schoutte, 1938) is taken to indicate poor capacity to regulate the shoot solute content, it should be noted that the absence of a casparian band in the ‘endodermoid’ layer of endohydric moss gametophytes (Scheiner, 1976) and in roots of Lycopodiaceae (Clarkson & Robards, 1975) does not have large effects on the shoot K/Na or K/Ca ratio relative to lower vascular plants possessing an endodermis (Walland & Kinzel, 1966), although the acquisition of solutes from leachate from leaves above them in the canopy may account for some of the high K/Na and K/Ca ratio in the shoots. Further work is clearly needed on the correlation between shoot solute content and the occurrence of a morphologically ‘normal’ endodermis (Grubb, 1967). The problems of regulating solute supply to the shoot of early vascular plants would have been greatly increased if the plants were living in a saline environment (see Church, 1919; Edwards el al., 1983). If the concentration of NaCl in the total plant water and the external solution is the same at, for example, 300 mol NaCl m-3, and the ratio of water transpired to water retained by the plant shoot during growth is 80 (Appendix 2), then the mean xylem concentration of NaCl must be only & of that in the soil solution. CONCLUSIONS It is hoped that this article has helped to relate the sequence of changes in the morphology and anatomy of plants seen in Silurian-Devonian fossils to physiological imperatives for the evolving vascular plants. However, it must be borne in mind that before the first known vascular plant (Cooksonia) and also subsequently, there was also a great variety of non-vascular terrestrial plants; not all of these were likely to have been closely related to the precursors of vascular plants (see Edwards, Bassett & Rogerson, 1979; Edwards, Edwards & Rayner, 1982; Edwards, Feehan & Smith, 1983). Eventually, competition with vascular plants and bryophytes extinguished these plants, leaving organisms which were obviously algae (including lichenized algae) and bryophytes as the only competitors with the homoiohydric vascular plants. The data most useful for ‘palaeoecophysiology’ which analysis of fossils can provide are (as seen in Appendices 1-3) the dimensions of xylem elements, the thickness of the cuticle, the stornatal frequency and size, the ratio of stornatal pore size to the size of the substomatal cavity (Pickard, 1982) and the ratio of internal to external area exposed to the gas phase. This is, of course, but a small fraction of the quantitative data available from the study of early vascular plant fossils, although the significance of the other data (e.g. the analysis by Bower (1930) of the surface/volume ratio of the stele) is less obvious in terms of today’s plant physiology. As mentioned in the Introduction, I do not propose to discuss the details of the evolution and significance of leaves, roots, heterospory, etc., 116 J. A. RAVEN which were later acquisitions by vascular plants (c( Raven, 1977a; Givnish, 1979). We may note that, as plants got larger, with greater bulk of nonphotosynthetic tissues to be produced and maintained, the rate of photosynthesis per unit of photosynthetic tissue required for growth becomes larger. Thus, as plants grew taller, with more conducting and structural tissue, they require more light to give adequate rates of photosynthesis. Thus large homoiohydric plants have higher light compensation points for growth than do many smaller homoiohydric plants, with the poikilohydric gametophytes (with essentially no non-green tissue) having even lower light compensation points (Raven & Beardall, 1982). Hence we can see how the diversity of plants, including herbs and gametophytes, could co-exist with forest trees in Upper Devonian and later forests (Phillips & Dimichelle, 1981). The forest trees had disposed, by abscission, of leaves or branches which were receiving so little light that they were below the whole-plant compensation point; this permits sufficient light to pass through the canopy to support the growth of plants of lower stature with lower light compensation points. ACKNOWLEDGEMENTS Dr Dianne Edwards and Professor W. G. Chaloner have provided very generous assistance with palaeobotany and morphology; errors which remain are entirely the responsibility of the author. APPENDIX 1: THE RELATIVE SPECIFIC H 2 0 CONDUCTANCE OF PARENCHYMA AND XYLEM TISSUES The units ofspecific H 2 0 conductance are m 2 s- 1 Pa- 1 (i.e. m 3 H 2 0 moving s __ , (m 2 area ofpathway normal to the H 2 0 flux)-' (Pa m-'ofdriving force in the direction of the H 2 0 flux)-'). Data discussed by Raven (1977a) suggest that the specific H 2 0 conductance of living parenchyma tissue (i.e. the apoplastic, cell wall pathway, involving no crossing of membranes, and the cellular pathway involving the crossing of at least two membranes per cell crossed, i.e. the plasmalemma at the 'influx' end and that at the 'efHux' end) is some w-IS m 2 s- 1 Pa- 1• Xylem consisting of tracheids has a specific conductance of some I0- 9 m 2 s-' Pa-', i.e. 10 6 times that of the parenchyma cell pathway. APPENDIX 2: THE H 2 0 LOSS ASSOCIATED WITH PHOTOSYNTHETIC C0 2 FIXATION IN A TERRESTRIAL PLANT The net photosynthetic rate of a plant can be expressed as: (I) PHYSIOLOGY AND FOSSIL PLANTS where Pnct Dco I ' 117 = C0 2 fixation rate/mol m- 2 s- 1 = Difussion coefficient of C0 2 in air/m 2 s- 1 = Diffusion path length from bulk air to the surface of the photosynthetic cell/m = C0 2 concentration in bulk air/mol m- 3 = C0 2 concentration at surface of photosynthetic cell/mol m- 3 The transpiratory water loss can similarly be expressed as: Tr = DHo ~ 1 (H 2 0] 1 -[H 2 0].) (2) = H 2 0 vapour transpired/mol m - 2 s- 1 =Diffusion coefficient of H 2 0 in air/m 2 s- 1 = Diffusion path from the surface of the photosynthetic cell to the bulk air/m [H 2 0]. = H 2 0 vapour concentration in bulk air/mol m - 3 [H 2 0Ji = H 2 0 vapour concentration at surface of photosynthetic cell/mol m - 3 where Tr Dividing equation (2) by equation (1): Tr _ DH,o ([H 2 0] 1 -[H 2 0].) Pnet- Dco, ([C0 2 ].-[C0 2 Ji) (3) With DH 0 = 1.6, air temperature leaf= temperature = 25°C, internal relative hum'idity of 99% ([H 2 0] 1 = 1270 mmol m-1, equivalent to a relatively low leaf 1/Jw of - 1.38 MPa), external relative humidity of 50% ([H 2 0].=640mmol m- 3 ), external C0 2 partial pressure of 30kPa ([C0 2 ] 8 = 10 mmol m- 3 ), internal C0 2 partial pressure of 20 kPa ([C0 2 ] 1 = 6.7 mmol m- 3 ), equation (3) shows that Tr (1270-640) -p = 1.6 = 302 mol H 2 0 lost per mol C0 2 fixed net ( 10- 617) For a plant with 50% C in the dry weight (Westlake, 1963), I mol C0 2 is equivalent to 24 g dry weight, while 302 mol H 2 0 has a mass of 5463 kg, so 227 g H 2 0 must be lost in gaining I g dry weight of photosynthate. Since dark respiration (see Raven, 1976) amounts to some 0.33 C lost as C0 2 per C assimilated into cell material in a C 3 herb, this brings the H 2 0 transpired per dry weight gain to 302 g H 2 0/g dry weight gain. In practice, C 3 plants lose at least 400 g H 2 0/g dry weight gain (e.g. Black, 1973). APPENDIX 3: THE SIGNIFICANCE OF EXPOSING A LARGE AREA OF CELL SURFACE TO THE GAS PHASE IN RELATION TO THE PLANT'S INVESTMENT IN RUBISCO AND IN STRUCTURE RUBISCO has a high Mr (5.5.10 5 ), a low solubility ( ~5 mol m- 3 ), a low specific reaction rate at C0 2 saturation at 25°C ( <20 mol C0 2 (mol enzyme)-' s-') and a Kt which is higher than the air-equilibrium C0 2 concentration in solution at 25°C (Kt> 10 mol m- 3, air-equilibrium solution= 10 mol m - 3 ) and, in addition to its carboxylase activity, it has an oxygenase activity which, in air-equilibrated solution, gives an oxygenase 118 J. A. RAVEN activity which consumes RuBP at about 0.25 of its rate of consumption by the carboxylase activity (Bird, Cornelius & Keys, 1981; Hall, Pierce & Tolbert, 1981; Lorimer, 1981; Raven, 1981a; Raven & Glidewell, 1981). The enzyme is conservative kinetically; within the eukaryotes the rate of CO, fixation in airquilibrated solution at 25°C varies (on a unit protein basis) only about two-fold between algae, bryophytes, pteridophytes, gynmosperms and angiosperms, with C,, C,, CAM or ‘CO, concentrating mechanism’ metabolism (see Cornelius & Keys, 1981; Jordan & Ogren, 1981; Lorimer, 1981; Raven & Beardall, 1981; Yeoh, Badger & Watson, 1981). Up to 25% of the protein (soluble and insoluble) in a photosynthetic cell is RUBISCO. Accordingly, economy in N and energy used for RUBISCO synthesis demands that the CO, and 0, concentrations at the site of RUBISCO activity should be as near the airequilibrated values as possible. Photosynthetic activity decreases CO, and increase 0, at the site of the enzyme (by almost equal absolute amounts), the changes increasing with increasing diffusive resistance to gas exchange between bulk air and RUBISCO (Raven, 1977a, 1981a; Raven & Glidewell, 1981; Samish, 1975). Since D, in water is only some lo-’ of DCO2in air, the adverse effect of 1 pm diffusive pathway in water on RUBISCO activity is equivalent to that of a 10 mm gas-phase diffusion path; the large M, and low solubility of RUBISCO means that RUBSICO added to a constant area of cellgas phase interface would be disadvantaged, in that the added RUBSICO would be further from the interface and thus be exposed to a lower absolute [CO,] and to a lower [CO,]/[O,]. At the other extreme, a minimal transport resistance (a monolayer of RUBISCO molecules at the water-gas interface, with optimal gas exchange between the interface and bulk air) would involve a large input of material (organic C) and energy into structures. Several lines of evidence (O’Leary, 1981; Wong, Cowan & Farquhar, 1979) suggest that, in extant C, plants, the diffusive resistance (a long gas-phase path through a medium with a high DCO,and a short liquid-phase path through a medium accounts for 0.2-0.5 of the total (lifecycle integrated) with a low D,,) limitation on photosynthesis; the remaining 0.5-0.8 of the limitation is related to biochemical processes (with RUBISCO a major contributor), This range may reflect a number of ‘local’ optima of the diffusive and biochemical limiting processes, granted various additional constraints of N supply, H,O availability, and the extent of respiratory losses (cf. Jones, 1976). In the context of early vascular plants, we may note particularly the following points: The enzyme RUBSICO was probably similar kinetically to the extant eukaryote enzymes, which, as has been pointed out above, are kinetically similar despite the substantial range of in vivo CO, and 0, concentrations which they encounter Uordan & Ogren, 1981; Lorimer, 1981; Yeoh et al., 1981). The absolute atmospheric CO, levels have been rather above the current levels, while the 0, level may have been 0.1-0.5 of the present atmospheric level, i.e. the ratio of carboxylase to oxygenase activity would have been higher than it is now for an enzyme with similar kinetic properties. Palaeozoic vascular plants were anatomically C, (rather than C,) plants; if we can take the carbon isotope ratio (6’,C) of Palaeozoic organic remains PHYSIOLOGY AND FOSSIL PLANTS 119 at face value (i.e. ignore diagenetic effects), then they were also physiologically C, plants (see O'Leary, 1981; Troughton, 1971). Some recent work on photosynthesis and transpiration in liverworts with (Murchunliu foliuceu Mitt.) and without (Moncleu forsteri Hook.) intercellular air spaces showed that the rate of photosynthesis on the basis of the external thallus area was no greater in Murchunliu than in Monoclea, but that the H,O loss per unit net photosynthesis was lower, under identical conditions, in Marchunliu than in Monoclea (Green & Snelgar, 1982). These findings suggest that economy of water use rather than enhanced net photosynthesis (on an external area basis) was a major early selective advantage of intercellular air spaces in terrestrial plants (cf. Pickard, 1982). however, other marchantiaceous species studied (Bjorkman & Gauhl, 1968; Fock, Krotkov & Canvin, 1969) have up to five times the light-saturated rate of photosynthesis in air than was reported for Marchantia foliuceu (all rates on an external area basis), thus suggesting that the increased area of photosynthetic cells exposed to the gas phase as a result of the occurrence of intercellular spaces in the Marchantiales can lead to rates of photosynthesis higher than could be achieved with a solid thallus. While the area of photosynthetic cell surface in contact with the gas phase is a very significant factor in determining the photosynthetic capacity of an organ in air at light saturation, the view (e.g. Nobel, 1977) that genotypic and phenotypic (e.g. in response to photon flux density for growth) variations in the photosynthetic rates of leaves (on an external area basis) disappear when the data are expressed on the basis of internal leaf area is clearly an over-simplification (e.g. Harvey, 1980; Raven & Glidewell, 1975). Accordingly, the area of (putatively) photosynthetic cells exposed to intercellular air spaces in fossil plants is probably not directly related to the capacity for photosynthesis, although it probably does indicate an upper limit (1-2 pmol CO, (m2 internal area)-' S K I ) , APPENDIX 4: THE SIGNIFICANCE OF XYLEM WATER TRANSPORT FOR SUPPLYING WATER TO CELLS PHOTOSYNTHESIZING WITH AIR AS THE CO, SOURCE For a plant with the dimensions of Eohostimella or Cooksoniu (i.e. an aerial axis 100 mm long and 1 mm in diameter) photosynthesizing at 1 pmol CO, (m2 aerial axis surface area)-' s-' (Nobel, 1977; Raven & Glidewell, 1981), the analysis of Appendix 2 shows that, with 227 g H,O lost per g dry weight gain, some 428 ng H,O s-' is lost by the photosynthesizing axis. If a linear gradient of water potential along the axis is assumed, a xylem-less plant such as Eohostimellu with a specific H,O conductance of lo-" m2 s-' Pa-' (Appendix 1) must have a $, at the apex of 27.3 MPa more negative than the $, at the base. For Cooksoniu, assuming a central xylem cylinder occupying 0.01 of the transverse areas of the axis (Raven, 1977a), and a xylem H,O conductance of lo-' m2 s-' Pa-', involves a JI, at the apex only 2.73 kPa more negative than that at the base. Since a $, of -27.3 MPa has a substantial inhibitory effect on the biochemistry of photosynthesis (Hsaio, 1973), there is very restricted scope ;, for Eohostimellu to extract H,O from a soil with a significant negative $ J. A. RAVEN 120 Cooksoniu could, by contrast, maintain its axis #w at a relatively high (not very negative) value, and thus permit photosynthesis, even when extracting H,O from a soil with a relatively negative #w. We may note that this rate of net CO, fixation, with a growth-associated rate of respiration equal to 0.25 of gross photosynthesis (Raven, 1976), 1/3 of the axis (50 mm length) underground, and a fresh weight/dry weight ratio of 10, gives a relative growth rate of 0.01 d-', i.e. an accumulation of dry matter which is only about twice the minimal maintenance respiration rate quoted by Penning de Vries (1975a). A ratio of internal to external exposed area of 10 would give, for a plant with intercellular air spaces and stomata, and the same dimensions as our idealized Eohostimella or Cooksoniu, a relative growth rate of 0.1 d-' if the photosynthetic rate on the basis of internal exposed area were 1 pmol CO, m-' s-I, i.e. a 20-fold excess of relative growth rate over specific maintenance rate. The enhanced rate of photosynthesis would imply an H,O loss in transpiration some ten times that for the Cooksoniu example (without intercellular air spaces) used earlier; with the same xylem dimensions and xylem specific conductance as in the earlier example the water flux would require a qW at the apex some 27.3 kPa more negative than that at the base. Woodhouse & Nobel (1982) present pertinent data on the xylem conductivity of extant leptosporangiate ferns. APPENDIX 5: T H E COSTS OF HOMOIOHYDRY AND DESICCATION-RESISTANCE Table 1 shows the degree of desiccation-tolerance of vegetative nonvascular and vascular plants. Intuitively, there could be costs (in terms of resource diversion) associated with both the homoiohydric and the desiccation-resistant mechanisms of surviving water shortage as vegetative plants. The xylem/cuticle/stomata/intercellular space system of the homoiohydric plant must divert resources from the production of more resource-acquiring machinery: data reviewed by Penning de Vries (1975b) show that lignin and lipids are intrinsically costly (energy per unit weight of product) to synthesize. The additional costs for a plant to be desiccation-resistant rather than desiccationsensitive are less readily quantifiable, but may involve cell compositions and Table 1. Taxonomic distribution of tolerance of desiccation in the vegetative state in extant non-vascular and vascular plants ~~~~ Tolerance of desiccation in the vegetative state Intolerant Tolerant Non-vascular plants Vascular plants Most of the algae which normally live fully submerged; many bryophytes and vascular plant free-living gametophytes of moist habitats. Many algae which are exposed to air for prolonged periods; most lichens; many bryophytes. Sporophytes (except those of resurrection plants) Sporophytes of a small fraction of herbaceous plants (resurrection plants) Data drawn from reviews by Bewley (l979), Gaff (l980), Hsaio (1973) and Levitt (1972). The extent to which the ability to withstand desiccation in the vegetative state correlates with the ability to withstand freezing (which can also act by desiccating cells) is not clear. PHYSIOLOGY AND FOSSIL PLANTS 121 architectures (e.g. a smaller degree of vacuolation) which are sub-optimal for resource acquisition, e.g. in terms of surface/volume relationship (Walter & Stadelmann, 1968), as well as the presence of repair mechanisms absent from desiccation-sensitive plants. The expression of these additional costs may be sought in the relative growth rate of the organisms, in that resources used for the homoiohydric mechanism or the desiccation-resistance mechanism cannot be used to produce more resourceacquisition apparatus. Comparisons here are very difficult: clearly organisms of similar size must be compared, in view of the lower relative growth rates found for larger organisms (see Blasco, Packard & Garfield, 1982). Comparing young ruderal or competitive strategy plants (see Grime, 1979; Raven, 1981b; Raven, Smith & Glidewell, 1979) we find that the highest reported relative growth rates are about 0.4 d-' for both marine algae (Jackson, 1980: table 111) and terrestrial vascular plants (Grime & Hunt, 1975). The data were obtained for light and nutrient saturation, natural inorganic carbon levels, and with the temperature for the land plant measurements higher than those for the algae. This latter difference would tend to underestimate the potential growth rate for the algae since, even if the lower temperatures were near optimal for the algae, there is a clear tendency for an increase in maximum relative growth rates with temperature for genotypes adapted to various temperatures (see Eppley, 1972). The consideration notwithstanding, the available data suggest that the maximum specific growth rate for the algae does not greatly exceed that of the vascular plants, despite the vascular plants having to bear the costs of homoiohydry while the algae (species of Laminaria and of Porphyra) with the highest growth rates are relatively desiccation-sensitive and so do not have to bear the (unquantified) cost of desiccation-resistance (cf. Fork & Oquist, 1981, who discuss the relative desiccation resistance of species of Porphyru). The relative growth rate of desiccation-tolerant poikilohydric plants which are otherwise comparable with the vascular plants and the desiccation-sensitive algae has not, to my knowledge, been shown to be as high as 0.4 d - '. We may note that the use of 'natural' inorganic carbon levels does not greatly disadvantage the terrestrial plant (with the stomata1 resistance between the source CO, and RUBISCO: Raven, 1981a) relative to the aquatic plants (where the greater diffusive resistance of water than of air tends to decrease the CO, availability to RUBISCO: Raven, 1970, 1981a; Raven, Beardall & Johnston, 1981; Smith & Walker, 1980). The growth rate data are inconclusive with respect to costs associated with the survival of water deficits in the vegetative state; further work aimed directly at this problem is needed to overcome the difficulties inherent in the promiscuous comparison of data in the analysis given above. The comparison is both possible and worthwhile. Analysis of the data in terms of the plants resistant to desiccation in their vegetative phase should certainly consider the costs of the sort of anti-photoinhibition mechanisms descussed by Sigfidsson & Oquist (1980) and Fork & Oquist (1981) (cf. Raven, 1981a), while it must be borne in mind with respect to vascular plants that even those which are not resurrection plants (i.e. able to tolerate vegetative dehydration) must have the genetic capacity for desiccation tolerance in their seeds. T h e analysis of data of this sort should not, of course, assume that the capacity for rapid growth is the only, or even the major, criterion of plant fitness! 5 J. A. RAVEN 122 REFERENCES ADDICOTT, F. T., 1982. Abscission (1st edition). Berkeley, California: University of California Press. BANKS, H. P., 1975. The oldest vascular plants: a note of caution. Reuiew of Palacobotany and Palynology, 20: 13-25. BECC, J. E., 1980. Morphological adaptations ofleaves to water stress. In N. C. Turner & P. J. Kramer (Eds), Adaptution of flants to Water and Hi h TemperatureStress: 33-42. New York: John Wiley. BEWLEY, J. D., 1979. Physiologica aspects of desiccation resistance. Annul Review .f Plant Physiology, 30: P 195-238. BIRD, 1. F., CORNELIUS, M. J. & KEYS, A. J., 1982. Affinity of RuBP carboxylases for carbon dioxide and inhibition of the enzyme by oxygen. JOUrnOl ofExpnimentu1 Botuny, 33: 1004-1013. BJORKMAN, 0.& GAUHL, E., 1968. Effect of temperature and oxygen concentration on photosynthesis in Marchuntiu polymorphu. rcorbook of the Camegie Institution of Washington, 67: 479482. BLACK, C. C., Jr., 1973. Photosynthetic carbon fixation in relation to net carbon dioxide uptake. Annual Review of Plant Physiology, 24: 253-286. BLASCO, D., PACKARD, T. T. & GARFIELD, P. C., 1982. Size dependence of growth rate, respiratory electron transport system activity, and chemical composition in marine diatoms in the laboratory. j'OUf'na1 of Phycology, 18: 58-63. BOLD, H. C. & WYNNE, M. J., 1977. Introduction to the A l p : Structure and Reproducfion (1st edition). Englewood Cliffs, New Jersey: Prentice Hall. BOWER, F. O., 1930. Size and Form in Plants. With Special ReJnmce to the Primury Conducting Tract. London: MacMillian & Co. BROWN, H. T. & ESCOMBE, F., 1900. Static diffusion of gases and liquids in relation to the assimilation of carbon and translocation in shoots. Philosophical Transactions of the Royal Society of London B, 193: 233-291. CAEMMERER, S. VON & FARQUHAR, G. C., 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. flantu, 153: 376-387. CANUTO, V. M., LEVINE, J. S., AUGUSTSSON, T. R. & IMHOFF, C. L., 1982. Ultraviolet radiation from the young sun and oxygen and ozone levels in the prebiological atmosphere. Nature, 296: 816-820. CHALONER, W. G., 1970. The rise of the first land plants. Biologicul Reuinus, 46: 353-377. CHALONER, W. G . & SHEERIN, A., 1979. Devonian macrofloras. In The Devonian $stem: Special Paper in P4l~OnlOhgy,23: 145-161. London: The Palaeontological Society. CHURCH, A. H., 1919. Thalassiophytu and the Subm'al Transmigration. Oxford Botanical Memoir Number 3. Oxford: Clarendon Press. CLARK, F. R. S. & RUSSELL, D. A., 1981. Fossil charcoal and the palaeoatmosphere. Nature, London,290: 428. [Letter commenting on Cope & Chaloner, 19801. CLARKSON, D. T. & ROBARDS, A. W., 1975. The endodermis, its structural development and physiological role. In J. C. Torrey & D. T. Clarkson (Eds), The Deuelopmmt and Function of Roots: 415-436. London: Academic Press. COPE, M. J. & CHALONER, W. G., 1980. Fossil charcoal as evidence of past atmospheric composition. Nature, London, 283: 647-649. COPE, M. J. & CHALONER, W. C., 1981. Fossil charcoal and the palaeoatmosphere. Nature, London, 290: 428. [Reply to letter from Clark & Russell, 1981.1 CORNER, D. J. H., 1964. The Lifc of Plants (1st edition). London: Weidenfeld & Nicolson. COWAN, I. R., 1977. Stomata1 behaviour and environment. Advunces in Botunical Research, 4: 117-228. DRAGSTEN, P. R., BLUMENTHAL, R. & HANDLER, J. S., 1981. Membrane asymmetry in epithelia: is the tight junction a bamer to diffusion in the plasma-membrane? Nature, London, 294: 718-722. EDWARDS, D., 1979. A late Silurian Flora from the lower Old Red Sandstone of south-west Dyfed. Palacontology, 22: 23-52. EDWARDS, D., 1982. Fragmentary non-vascular plant microfossils from the late Silurian of Wales. Botunical J o u d of the Linnean Society, 84: 223-256. EDWARDS, D., BASSETT, M. G. & ROGERSON, E. C. W., 1979. The earliest vascular land plants: continuing the search for prooC Lcthuia, 12: 313-324. EDWARDS, D., EDWARDS, D. S. & RAYNER, R., 1982. The cuticles of early vascular plants. In The Plunf Cuticle: 341-346. London: Academic Press. EDWARDS, D., FEEHAN, J. & SMITH, D. G . , 1983. A late Wenlock flora from Co. Tipperary, Ireland. Botanical Journal of the Linneun Society, &: 19-36. EDWARDS, D. S. & LYON, A. C., 1983. Algae from the Rhynie Chert. Eotunical Journul of Lhe Linnean Society, 86: 37-55. EPPLEY, R. W., 1972. Temperature and phytoplankton growth. Fishery Bulletin, 70: 1063-1085. FOCK, H., KROTKOV, G. & CANVIN, D. T., 1969. Photorespiration in liverworts and leaves. In H. Metzner (Ed.), Progress in Photosynthetic Research, I : 482-487. Tubingen International Biological Union. FORK, D. C. & OQUIST, C., 1981. The effects of desiccation on excitation energy transfer at physiological temperatures between the two photosystems of the red alga Porphya perjoratu. <eitschrii fur PpantmPhysiologie, 104: 385-394. FRITSCH, F. E., 1945. Studies on the comparative morphology of the algae. IV. Algae and archegoniate plants. Annals of Botuny (New Series), 9: 1-30. PHYSIOLOGY AND FOSSIL PLANTS I23 GAFF, D. F., 1980. Protoplasmic tolerance of extreme water stress. In N. C. Turner & P. J. Kramer (Eds), Adaptation of Plant to Water and High Temperature Stress: 207-230. New York: John Wiley. GARLAND, P. B., 1981. The evolution of membrane-bound bioenergetic systems: the development of vectorial oxidoreductions. Sympoia of the Society for General Microbiology, 32: 273-283. GIVNISH, T., 1979. On the adaptive significance of leaf form. In 0. T.Solberg, S. Jain, G . B. Johnson & P. H. Raven (Eds), Topics in Plant Population Biology: 375-407. Columbia: Columbia University Press. GIVNISH, T. J.. 1982.On the adaptive significance of leaf height in forest herbs. The Ameri'can Naturalist, 120: 353-381. GOMES, C. R. M. & GOTTLEIB, 0. R., 1978.The evolution ofstructural biopolymers is connected? Revista brasiliera & Botanica, I : 41-47. GORHAM, E., VITOUSEK, P. M. & BEINERS, W. A., 1979.The regulation of chemical budgets over the course of terrestrial ecosystem succession. Annual Review of Ecology and Systematics, 10: 53-84. GRAHAM, L. E., 1982.The occurrence, evolution and phylogenetic significance of parenchyma in Coleochaete Breb (Chlorophyta). American Journal of Bokmy, 69: 447-454. GRAHAM, L. E. & WILCOX, L. W., 1981. Placental transfer cells in the green alga Cokochacte. Journal of Phycology, 17: 5s. GRAMBAST, L. J., 1974. Phylogeny of the Charophyta. 'Taxon, 23: 463-481. GREEN, T. G . A. & SNELGAR, W. P., 1982. A comparison of photosynthesis in two thalloid liverworts. Oecologia, 54: 275-280. GRIME, J. P., 1979. Plants Stratcgies ~ n dVegetational Processes (1st edition). Chichester: John Wiley. GRIME,J. P. & HUNT, R., 1975.Relativegrowth rate: itsrangeand adaptivesignificanceinalocal flora.Journalof Ecology, 63: 393-422. GROSS, G. G . , 1980. The biochemistry of lignification. Advances in Botanical Research, 8: 26-63. GRUBB, P. J., 1967. Uptake and Movement ofSalts in Polytrichum, Ph.D. Thesis, University of Cambridge. GRUBB, P.J., 1977.Control offorest growth and distribution on wet tropical mountains, with special reference to mineral nutrition. Annual Review of Ecology and Systmatics, 8: 83-107. GUNNING, B. E. S., PATE, J. S. & GREEN, L. W., 1970.Transfer cells in the vascular system of stems: taxonomy, association with nodes, and structure. Protoplasma, 71: 147-1 71. HADLEY, N. F., 1981. Cuticular lipids of terrestrial plants and arthropods: a comparison of their structure, composition and water-proofing function. Biological Reviews, 56: 23-47. HALL, N. P., PIERCE, J. & TOLBERT, N. W., 1981. Formation of a carboxyarabinitol bisphosphate complex with ribulose bisphosphate carboxylasc/oxygcnase and theoretical specific activity of the enzyme. Archives of Biochemistry and Biophysics, 212: 1 15-1 19. HARVEY, G. W., 1980. Photosynthetic performance of isolated cells from sun and shade plants. rearbook of the Carnegie Institution of Washington, 79: 160-164. HOLLAND, H. D., 1965. The history of ocean water and its effect on the chemistry of the atmosphere. Proceedings of the National Academy of Sciences, 53: 1173-1 182. HSAIO, T. C., 1973.Plant responses to water stress. Annual Review of Plant Physiology, 24: 519-570. JACKSON, G. A., 1980. Marine biomass production through seaweed aquaculture. In A. San Pietro (Ed.), Biochemical and Photosynthetic Aspects of Energy Production: 31-58. New York: Academic Press. JONES, H. G., 1976. Crop characteristics and the ratio between assimilation and transpiration. Journal of Applied Ecology, 13: 605-622. JONES, H. G . & HIGGS, K. H., 1980. Resistance to water loss from the mesophyll cell surface in plant leaves. Journal of Experimental Botany, 40: 545-554. JONES, H. G. & NORTON, T. A., 1979. Internal factors controlling the rate of evaporation from fronds of some intertidal algae. N e w Phytologist, 83: 771-782. JORDAN, D. B. & OGREN, W. L., 1981. Species variation in the specificity of ribulose bisphosphate carboxylase-oxygenase. Nature, London, 219: 513-5 15. KEDDY, P. A., 1981. Why gametophytes and sporophytes are different: form and function in a terrestrial environment. American Naturalist, 118: 452-454. KIDSTON, R. & LANC, W. H., 1917.On old red sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part I. Rhynio g w y u u - v a u g h i i , Kidston and Lang. 'Trunsactim of fhe Royal Society of Edinburgh, 51: 761-784. KLEIN, R. H., 1978.Plants and near-ultraviolet radiation. Botanical Reviews, 44: 1-127. KRASSILOV, V., 1981. Orestovia and the origin of vascular plants. Lethaia, 14: 235-250. LAMONT, H. C., 1969. Sacrificial cell death and trichome breakage in an Oscillatoriacean blue-green alga: the role of murein. Archiv fur Mikrobiologie, 69: 237-259. LANG, A., 1977.A model of mass flow in the phloem. Australian Journal of Plant Physiology, 5: 535-546. LARCHER, W., 1979.Physiological Plant Ecology (2nd edition). Berlin: Springer. LENDZIAN, K. J., 1982.Gas permeability of plant cuticles. Oxygen permeability. Plan&, 155: 310-315. LEVITT, J., 1972.Responses of Plants to Environmental Stresses, (1st edition). New York: Academic Press. LORIMER, G . H., 1981. The carboxylation and oxygenation of ribulose 1,5-bisphosphate: the primary events in photosynthesis and photorespiration. Annual Review of Plant Physiology, 32: 349-383. LOVELOCK, J. E. & WHITFIELD, M.,1982.Life span of the biosphere. Nahrc, London, 2%:561-563. LOWRY, B., LEE, D. & HEBANT, C., 1980.The origin ofland plants: a new look at an old problem. 'Taxon, 29: 183-197. J. A. RAVEN 124 LUTHER, H., 1949. Vorschlag zu einer okoloischen Grundeinte ling der Hydrophyten. Acfa Eofunica Fmnica, 44: 1-15. MABBERLEY, D. J,, 1981. Revolufionury Botany (1st edition). Oxford: Clarendon Press. MARGULIS, L., 1981. Symbiosis in Cell Evolution. San Francisco: W. H. Freeman and Company. MARTIN, J. F. &JUNIPER, B. E., 1970. The Cuficles of Plants (1st edition). London: Edward Arnold. MEIDNER, H.& MANSFIELD, T. A., 1968. Physiology ofStomafu (1st edition). London: McGraw-Hill. MELKONIAN, M., 1982. Structural and evolutionary aspects of the flagellar apparatus in green algae and land plants. Tuxon, 31: 255-256. METTING, B., 1981. The systematics and ecology of soil algae. Eofunical Reviews, 47: 195-312. MORRIS, 1. (Ed.),1980. The Physiological Ecology of Phylopldton (1st edition). Oxford: Blackwell Scientific Publications. MOSS, B., 1980. Ecology of Freshwafers (1st edition). Oxford: Blackwell Scientific Publications. NEFTEL, A., OESCHEGER, H.,SCHWANDER, J., STAUFFER, B. & ZUMBRUMM, R., 1982. Ice core sample measurementsgiveatmosphericCO, content during the past40,OOOyears.Nature,London,295: 220-223. NEWMAN, M. J., 1980. The evolution of the solar constant. Originr ofL@, 10: 105-1 10. NIKLAS, K. J., 1978. Morphometric relationships and rates of evolution among Paleozoic vascular plants. Evolufionury Biology, 11: 509-543. NIKLAS, K. J. & PRATT, L. M., 1980. Evidence for lignin-like constituents in early Silurian (Llandoverian) plant fossils. Science, 209: 396-398. NOBEL, P. S., 1974. An Infroducfion fo Eiophysicul Planf Physiology (1st edition). San Francisco: W. H. Freeman and Company. NOBEL, P. S., 1977. Internal leaf area and cellular CO, resistance: photosynthetic implications of variations with growth conditions and plant species. Physiologia Planfurum, 40: 137-144. NOBEL, P. S., 1980. Leaf anatomy and water use efficiency. In N. C. Turner & P. J. Kramer (Eds), Adapfufion of Planfs to Wafer and High Temperature Stress: 43-55. New York: John Wiley. NYE, P. H. & TINKER P. B., 1977. Solufc MoumMf in fhe Soil-Roof $stem (1st edition). Oxford: Blackwell Scientific Publications. OLEARY, M. H.,1981. Carbon isotope fractionation in plants. Phyfochemistry, 20: 553-568. OLIVER, S. & BARBER, S. A,, 1966A. An evaluation of the mechanisms governing the supply of Ca, Mg, K and Na to soybean roots (Glyciw m a ) . Soil Science Socieg of A m ' c a , Proceedings, B: 82-86. OLIVER, S. & BARBER, S. A., 19668. Mechanisms for the movement of Mn, Fe, B, Cu,An, Al and Sr from soil to the surface of soybean roots (Clycinc mux). Soil Science Sm'efy of A m ' c a , Proceedings, B: 468-470. OSMOND, C. B., BJORKMAN, 0. & ANDERSON, D. J., 1980. Physiological Processes in Planf Ecology: Towardr a Synthesis wifh Afriplex (1st edition). Berlin: Springer-Verlag. PASSIOURA, J. B., 1976. Translocation and the diffusion equation. In I. F. Wardlaw & J. B. Passioura (Eds), Trunsporf and Trunsfm Processes in Planfs: 357-361. New York: Academic Press. PATE, J. S., 1980. Transport and partitioning of nitrogenous solutes. Annual Review of Planf Physiology, 31: 3 13-340. PATE, J. S. & LAYZELL, D. B., 1981. Carbon and nitrogen partitioning in the whole plant-a thesis based on empirical modelling. In J. D. Bewley (Ed.), Nitrogen and Carbon Mehbolism: 94-134. The Hague: Dr W. Junk, Publisher. PENNING DE VRIES, F. W. T., 1975A. The cost of maintenance processes in plant cells. Annuls of Botany, 39: 77-92. PENNING DE VRIES, F. W. T., 1975b. The use of assimilates in higher plants. In J. P. Cooper (Ed.), Photosynthesis and Productivi& in Diymmt Environments: 459-480. Cambridge: Cambridge University Press. PHILLIPS, T. L. & DIMICHELE, W. A., 1981. Palaeoecology ofmiddle Pennsylvanean age coal swamps in southern Illinois-Hemn Coal member at Sahara Mine No. 6. In K. J. Niklas (Ed.), Pulueobofuny, Pulueoecology and Euolufion, Vol. I : 231-284. New York: Praeger. PICKARD, W. F., 1981. The ascent of sap in plants. Progress in EiopAyscs and Molecular Biology, 37: 181-229. PICKARD, W. F., 1982. Why is the substomatal chamber as large as it is? Plant Physiology, 69: 971-974. PITMAN, M. G., 1977. Ion transport into the xylem. Annual Rcuiew ofPlan1 Physiology, 28: 71-88. PRENZEL, J., 1979. Mass flow to the root system and mineral uptake of a beech stand calculated from threeyear field data. Plant and Soil, 51: 39-49. PROCTOR, M. C. F., 1979. Structure and eco-physiological adaptation in bryophytes. In G. C. S. Clarke & J. G. Duckett (Eds), Eryophyte Sysfemutics: 479-509. London: Academic Press. RAVEN, J. A., 1970. Exogenous inorganic carbon sources in plant photosynthesis. Eiological Reviews, 45: 167-221. RAVEN, J. A., 1976. The quantitative role of 'dark' respiratory processes in heterotrophic and photolithotrophic plant growth. Annuls of Eofuny, 40: 587-602. RAVEN, J. A., 1977a. The evolution of vascular land plants in relation to supracellular transport processes. Advances in Eo&anUd Research, 5: 153-2 19. RAVEN, J. A., 1977b. H + and Caz+ in phloem and symplast: relation ofnlative immobility of the ions to the cytoplasmic nature of the transport paths. New Phytologisf, 79: 465-480. RAVEN, J. A., 1980. Nutrient transport in micrvalgae. Aduances in Microbial Physiology, 21: 47-226. RAVEN, J. A., 1981a. Ribulose bisphosphate carboxylase activity in terrestrial plants: significance of 0, and CO, diffusion. In H. Smith (Ed.), Conmenfuries in Planf Science, Vol. 2: 27-39. Oxford: Pergamon Press. PHYSIOLOGY AND FOSSIL PLANTS 125 RAVEN, J. A., 1981b. Nutritional strategies of Submerged benthic plants: the acquisition of C, N and P by rhizophytes and haptophytes. New Phytologisf, 88: 1-30, RAVEN, J. A., 1983a. The transport and function ofsilicon in plants. Biological Reviews, 58: 179-207. RAVEN, J. A,, 1983b. Phytophages of xylem and phloem: a comparison of animal and plant sap-feeders. Advances in Ecological Research, 13: in press. RAVEN, J. A. & BEARDALL, J., 1981. Respiration and photorespiration. In T. Platt (Ed.). Physiological Buses of Phytoplankton Ecology: 55-82. Canadian Journal Bulletin of Fisheries and Aquatic Science No. 210. RAVEN, J. A. & BEARDALL, J., 1981. 'The lower limit of photon fluence rate for phototrophic growth: the significance of 'slippage' reactions. Plant, Cell and Environment, 5: 13-26. RAVEN, J. A,, BEARDALL, J. & J O H N S T O N , A. M., 1981. Inorganic carbon transport in relation to H transport at the plasmalemma of photosynthetic cells. In D. Marme, E. Marre and R. Hertel (Eds), Plasmalemma and Tonoplat: Their Functions in the Plant Cell: 41-47. Amsterdam: Elsevier. RAVEN, J. A. & GLIDEWELL, S. M., 1975. Photosynthesis, respiration and growth in the shade-adapted alga Hydrodicyton africanum. Photosynthetica, 9: 36 1-37 1. RAVEN, J. A. & GLIDEWELL, S. M., 1981. Processes limiting photosynthetic conductance. In C. B. Johnson (Ed.), Physiological Processes Limiting Plant Productiuip: 109- 136. London: Butterworths. RAVEN, J. A. & SMITH, F. A., 1976. Nitrogen assimilation and transport in vascular land plants in relation to intraccllular pH regulation. New Phytologist, 76: 205-2 12. RAVEN, J. A,, SMITH, F. A. & GLIDEWELL, S. M., 1979. Photosynthetic capacities and biological strategies of giant-celled and small-celled macro-algae. New Phytologist, 83: 285-291. RAVEN, J. A,, SMITH, F. A. & SMITH, S. E., 1980. Ions and osmoregulation. In D. W. Rains, R. C. Valentine & A. Hollaender (Eds), Genetic Engineering of Osmoregulation: Impact on Plant Productiuip for Food, Chemicals and Energy: 101-1 18. New York: Plenum Press. RAVEN, J. A,, SMITH, S. E. & SMITH, F. A., 1978. Ammonium assimilation and the role of mycorrhizas in rlimax communities in Scotland. Transactions of the Botanical Sociep of Edinburgh, 43: 27-35. REDFIELD, A. C., 1958. T h e biological control of chemical factors in the environment. American Scientist, 46: 204-22 I . ROLAND, J. C.. 1978. Cell wall differentiation and stages involved with intercellular air space opening. ~ournalof Cell Science, 32: 325-336. RU'MPF, G., 1904. Rhizodermis, Hypodermis und Endodermis der farnwurzel. Bibliotheca Botanica, 62: 1-48. SAMISH, Y. B., 1975. Oxygen build-up in photosynthesing leaves and canopies is small. Photosynthetica, 9: 372-375. SCHEINER, D. C., 1976. Some fine structural observations on the rhizome of Dendrolignotrichum (Bryophyta). Protoplasmu, 89: 323-337. SCHONHERR, J. & MERIDA, T., 1981. Water permeability of plant cuticular membranes: the effects of humidity and temperaturc on the permeability of isolated cuticles of onion bulb scales. Phnl, Cell and Environment, 4: 349-354. SCHOPF, J. M., MENCHER, E., BOUCOT, A. J. & ANDREWS, H. N., 1966. Erect plants in the early silurian of Maine. U.S. C~eologicalSuruey Professional Paper, 550-D:D69-D75. SCHOU'I'E, J. C., 1938. Anatomy. In F. Verdoorn (Ed.), Mannual of Pteridology: 65-104. T h e Hague: Martinus Nijhoff. SCOTT-RUSSELL, R., 1977. Planf Hoot System: Their Function and Interaction with Soil (1st edition). Maidenhead: McGraw-Hill. SIGFIDSSON, B. & OQUIST, G . , 1980. Preferential distribution of excitation energy into photosystem one of desirratrd samples of the lichen Cladonia implcxa and the isolated lichen-alga Trebouxia pyriyormis. Physiologia Plantarum, 49: 329-335. SMITH, F. A. & WALKER, N. A., 1980. Photosynthesis by aquatic plants: effects of unstirred layers in relation to assimilation of CO, and HCO, and to carbon isotope discrimination. New Phytologist, w6: 245-259. SMITH, S. E., 1980. Mycorrhizas of autotrophic higher plants. Biological Reviews, 55: 475-510. SPORNE, K. R., 1974a. The Morphology of Angiosperms, (1st edition). London: Hutchinson. SPORNE, K. R., 1974b. The Morphology of Gymnosperms, (2nd edition). London: Hutchinson. SPORNE, K. R., 1975. The Morphology of Pteridophytes, (4th edition). London: Hutchinson. S'I'EBBINS, G . I,. & HILL, C. J. C., 1980. Did multicellular plants invade the land? American Naturalist, 115: 342--353. SILWAR'T, K. D. & M A I T O X , K. R., 1978. Structural evolution in the flagellated cells of green algae and land plants. Biosystem, 10: 145-152. STUBBLEFIELD, S. tk BANKS, H. P., 1978. The cuticle of Drepunophycus spinueJormis, a long-ranging Devonian lycopod from New York and Eastern Canada. American Journal of Botany, 65: 110-1 18. SWAIN, T., 1978. Plant-animal coevolution; a synoptic view of Palaeozoic and Mesozoic. In J. B. Harborne (Ed.), Biochemical Aspects of Plant and Animal Co-evolution: 3-19. London: Academic Press. SWAIN, 'I'. & COOPER-DRIVER, G., 1981. Biochemical evolution of early land plants. In K. J. Niklas (Ed.), Palaeobotuny, Palaroecology and Evolution, Vol. I : 103-1 14. New York: Praeger. TAYLOR, T. N., 1982. T h e origin of land plants: a palaeobotanical perspective. Taxon, 31: 155-177. TROUGHTON, J. H., 1971. Aspects of the evolution of the photosynthetic carboxylation reaction in plants. In M. D. Hatch, C. B. Osmond & R. 0. Slayter (Eds), Phofosynthesis and Photorespiration: 124-129. New York: John Wiley. 126 J. A. RAVEN - WALLAND. A. & KINZEL.. H.,. 1966. Uber die Zusammensetzuna der Zellsafte bei ArcheKoniaten. Flora, 156: 597633. WALTON, J., 1940.An Introduction to the Stuay of Fossil Plants, (1st edition). London: A. C. Black. WALTON, J., 1964. On the morphology of <osfcrophyllum and some other Devonian plants. Plyfomorphology, /4: 155-lfio. . .. . - - .- -. WALTER, H. & STADELMA”, E. J., 1968. The physiological prerequisites for the transition of autotrophic plants from water to terrestrial life. EiosCimtr, 18: 694-701. WESTLAKE, D. F., 1963. Comparisons of plant productivity. Biological Reviews, 38: 385-425. WETHERBEE, R. & KRAFI‘, G. T., 1981. Morphological and fine structural features of pit connections in Ctypfonaia sp., a highly differentiated marine red alga from Australia. Profoplama, 1M: 167-172. WHATLEY, J. M., 1982. Ultrastructure of plastid inheritance: green algae to angiosperms. Biological Reviews, 57: 527-569. WOODHOUSE, R. M. & NOBEL, P. S., 1982.Stipe anatomy, water potentials and xylem conductances in seven species of ferns (Filicopsida). A m c a n Journal of Botany, 69: 135-140. WONG, S. C.,COWAN, I. R. & FARQUHAR, G. D., 1979. Stomata1 conductance correlates with photosynthetic capacity. Nature, London, 282: 424-426. YEOH, H-H., BADGER, M. R. & WATSON, L., 1981. Variations in kinetic properties of ribulose bisphosphate carboxylase among plants. Plant Physiology, 67: 1 I5I - I 155.