* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
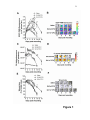
Download Changes in endothelial phenotype may explain changes in
Extracellular matrix wikipedia , lookup
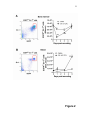
List of types of proteins wikipedia , lookup
Cell culture wikipedia , lookup
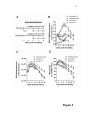
Cellular differentiation wikipedia , lookup
Cell encapsulation wikipedia , lookup
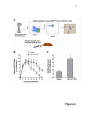
Organ-on-a-chip wikipedia , lookup
Tissue engineering wikipedia , lookup
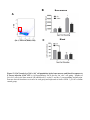
1 Neutrophil survival and c-kit+ progenitor proliferation in Staphylococcus aureus infected skin wounds promote resolution Min-Ho Kim1*, Jennifer L. Granick2*, Cindy Kwok1, Naomi J. Walker2, Dori L. Borjesson2, Fitz-Roy E. Curry3, Lloyd S. Miller4, Scott I. Simon1 1 Department of Biomedical Engineering, University of California at Davis, Davis, CA 95616 USA 2 Department of Veterinary Medicine: Pathology, Microbiology, Immunology, University of California at Davis School of Veterinary Medicine, Davis, CA 95616 USA 3 Department of Physiology and Membrane Biology, University of California at Davis School of Medicine, Davis, CA 95616 USA 4 Division of Dermatology, University of California at Los Angeles, Los Angeles, CA 90095 USA *Authors contributed equally to the manuscript. Running title: Neutrophil survival and proliferation Corresponding author: Scott I. Simon Department of Biomedical Engineering University of California at Davis 451 E. Health Sciences Dr. Davis, CA 95616 Phone: 530-752-0299 Fax: 530-754-5739 E-mail: [email protected] 2 Abstract Polymorphonuclear neutrophils (PMNs) are critical for the formation, maintenance and resolution of bacterial abscesses. However, the mechanisms that regulate PMN survival and proliferation during the evolution of an abscess are not well defined. Using a mouse model of Staphylococcus aureus-abscess formation within a cutaneous wound combined with real time imaging of genetically tagged PMNs, we observed that a high bacterial burden elicited a sustained mobilization of PMN from bone marrow to the infected wound where their lifespan was markedly extended. A continuous rise in wound PMN number that was not accounted for by trafficking from the bone marrow or prolonged survival correlated with homing of c-kit+ progenitor cells from blood to the wound where they proliferated and formed mature PMNs. Furthermore, we confirmed that progenitor cells are not only important contributors to PMN expansion in the wound but also functionally important for immune protection by blocking their recruitment with an antibody to c-kit. This severely limited proliferation of mature PMNs in the wound and mouse survival. We conclude that the abscess environment provides a niche capable of regulating PMN survival and local proliferation of bone marrow-derived c-kit+ progenitor cells. 3 Introduction Neutrophil or polymorphonuclear cell (PMN) abscess formation is a critical event in the innate immune response against invading pathogens and is required for wound resolution and bacterial clearance1,2. This is especially the case during Staphylococcus aureus (S. aureus) infections, where PMNs are recruited and form an abscess at the site of infection to promote bacterial clearance3-5. Applying real-time fluorescence imaging of a full thickness skin wound in mice possessing PMNs engineered to produce enhanced green fluorescence protein (EGFP-PMN), we previously reported that the numbers of PMNs recruited to the wound bed doubles in response to infection with S. aureus or peritoneal injection of GM-CSF4. These studies revealed the dynamics of PMNs entering the circulation from the bone marrow pool that enabled them to concentrate in the wound at a density up to 40-fold above that in the blood. Thus, inflammation and infection provide dual signals for the rapid mobilization of PMNs from the bone marrow compartment and recruitment to the site of tissue insult6,7. However, the mechanisms that regulate this feedback and maintain numbers sufficient to resolve infection while avoiding tissue damage are not entirely known. PMNs are attracted from the circulation to a site of infection or inflammation by CXC chemokines, including the IL-8 homologues keratinocyte chemoattractant (KC) and macrophage inflammatory protein-2 (MIP-2), which are produced by murine endothelium, macrophages, epithelial cells and fibroblasts8-10. In addition, we found that other signals, including IL-17, are critical for the production of KC and MIP-2 and subsequent recruitment of PMNs to a site of cutaneous S. aureus infection11. PMNs also generate paracrine signals that regulate their numbers in the wound including TNF-α and IL-1, 4 which promote mobilization of PMNs from the large storage pool in the bone marrow12-14. Although mature PMNs are reported to have a half-life in the circulation of only ~12 hours15,16, inflammatory cytokines can prolong PMN survival in vitro17. Whether this prolonged survival occurs in vivo during abscess formation is unknown. Cytokines produced within infected wounds may also influence the survival of PMN within the abscess by down regulating apoptosis18,19. It is well established that PMN numbers can be rapidly expanded in the bone marrow from lineage negative and c-kit receptor positive (Lin- c-kit+) progenitor cells. Inflammatory stimuli can induce direct mobilization and infiltration of Lin - c-kit+ cells to peripheral sites20,21. Furthermore, Lin- c-kit+ cells can be activated by Toll-like receptor (TLR) agonists to trigger proliferation and rapid myeloid differentiation in vitro and in vivo22,23. Based on these data, we hypothesized that PMN abscess formation and maintenance involves prolonged PMN survival within the abscess and the local generation of PMNs from myeloid progenitor cells present both in the bone marrow and at the site of the infection. Materials and Methods Mice EGFP-lysozyme M (lys) knock in-mice (EGFP-lys-mice)24 (kind gift from Dr. Thomas Graf) were backcrossed for 10 generations onto a C57BL/6 background (Jackson Lab, Bar Harbor, ME) in an animal facility at University of California at Davis. Male mice between 8 and 14 weeks of age were used in all the experiments. All animal experiments were approved by Institutional Animal Care and Use Committee (IACUC) of the 5 University of California at Davis and performed following the guidelines of Animal Welfare Act and the Health Research Extension Act. Mouse model of S. aureus wound infection Mouse skin wounding and S. aureus infection were performed as described previously4 and in the Supplemental Methods. Non-invasive quantification of wound EGFP-PMNs trafficking The trafficking of EGFP-PMNs at wound sites over time was determined non-invasively using the Xenogen IVIS 100 (Xenogen Inc., CA) as described previously4 and in the Supplemental Methods. Preparation and in vivo bioluminescent imaging of S. aureus Bioluminescent strain (SH1000) of S. aureus13 was prepared and inoculated into wound site, and actively metabolizing S. aureus was visualized using Xenogen imaging system as described previously4 and in the Supplemental Methods. Collection of bone marrow cells and blood samples For murine bone marrow cell collection, mice were humanely euthanized with CO2 at selected time points and the femurs from both hind limbs were removed. Bone marrow cells were flushed from each bone with HBSS, pelleted and then resuspended with buffer for further experiments. Mouse whole blood was drawn via cardiac puncture into EDTA and red blood cells were depleted with red cell lysis buffer (Biolegend, CA). The total 6 numbers of bone marrow and blood leukocytes were counted using an automatic cell counter (Coulter counter, Beckman Coulter Inc., CA). Immuno-depletion of systemic PMNs and c-kit progenitors Systemic PMNs were depleted by multiple injections (i.p) of anti-Gr-1 monoclonal antibody (RB6-8C5 clone, 0.1 mg each injection, eBioscience, CA). For early and sustained depletion of PMNs (pre Gr-1), mice were treated with Gr-1 antibody or isotype control rat IgG (eBioscience, CA) every other day, starting 24 h before wounding up to day 5. For delayed depletion of PMNs (post Gr-1), mice were injected with Gr-1 antibody every other day, starting 24 h after wounding up to day 5. For c-kit+ progenitor cell depletion, 1 mg of the anti-c-kit antibody ACK2 (University of California-San Francisco Hybridoma and Monoclonal Antibody Core, CA) or isotype control rat anti-mouse IgG was given (i.p.) 5 days prior to wounding and again 1 day after wounding. Flow cytometric immunophenotyping assay Flow cytometric immunophenotyping assay to characterize mature PMN and Lin- c-kit+ cells were performed using bone marrow and blood cells collected from EGFP-lys-mice as described in Supplemental Methods. FACS cell sorting Bone marrow cells isolated from EGFP-lys-mice were sorted into c-kit+ progenitors and mature EGFP-PMNs using the MoFlo cell sorter (Beckman Coulter, Inc., CA) as described in Supplemental Methods. 7 Tissue half-life of PMNs Neutrophil tissue half-life was determined by quantifying the rate of PMN (c-kitEGFPhigh Gr-1high CD11bhigh phenotype) clearance from sites of wound infection as described in Supplemental Methods. In vivo assessment of c-kit+ progenitor cell trafficking in wounded skin FACS sorted EGFP- c-kit+ cells from EGFP-lys-mouse bone marrow (5x105 cells in 0.1 mL sterile saline) were fluorescently labeled with Qtracker 705® (Invitrogen, CA) and then injected via tail vein into C57BL/6 mice 3hr after wounding and S. aureus (1x107 CFU) (or sterile saline) treatment on wounded skin. At 24 h post-transfer, Qtracker 705expressing EGFP- c-kit+ cells within the wound area were visualized using the Xenogen system (excitation at 445~490 nm and emission at 695~770 nm) at an exposure time of 1 s. In vivo assessment of c-kit+ progenitor cell differentiation to EGFP-PMN in wounded skin The FACS sorted EGFP- c-kit+ cells from EGFP-lys-mouse bone marrow (5x105 cells in 0.1 mL sterile saline) were intravenously injected via tail-vein to C57BL/6 mice. Using the Xenogen imaging system, the proliferative capacity of EGFP- c-kit+ cells to differentiate into EGFP-PMNs was assessed by detecting time-dependent increases in the EGFP fluorescence signal. Following adoptive transfer of EGFP- c-kit+ cells, circulating EGFP-PMN were depleted by repetitive treatment with Gr-1 antibody at days 1, 3, and 5 8 to block the potential contribution of EGFP-PMNs emigrating into tissue from outside wounded skin. Colony forming unit assay In order to quantify the proliferative capacity of cells isolated from bone marrow and wounded skin of EGFP-lys-mice, colony forming unit assays were performed in methylcellulose media supplemented with cytokines to support growth of myeloid progenitors (Methocult GF M3434, Stem Cell Tech, Vancouver, Canada) at a cell density of 3x105/mL. Detailed methods are described in Supplemental Methods. Mathematical modeling to estimate tissue PMN number during S. aureus infection A mathematical model was developed to predict the kinetics of PMN influx, survival, and production during S. aureus infection and is described in detail in Supplemental Methods. Statistical Analysis Data analysis was performed using GraphPad Prism version 5.0 software (GraphPad Software, San Diego, CA). Statistical significance between two groups was determined by two-tailed unpaired t tests. Mice survival analysis was performed based on Log-rank test. P values of <0.05 were considered statistically significant. 9 Results S. aureus infection of skin wounds increase PMN trafficking and mobilization from bone marrow. To investigate the dynamics of PMN recruitment to S. aureus-infected cutaneous wounds, the degree of PMN infiltration was measured in wounds infected with a low (2x106 CFUs) or a high (1x107 CFUs) inoculum and compared to mice treated with a saline vehicle control. Whole animal fluorescence imaging of transgenic mice expressing the lysozyme-EGFP gene (EGFP-lys-mice) was used to quantify EGFP-PMN infiltration4, while luminescence provided enumeration of a bioluminescent strain of S. aureus11 within the wound bed. During the initial 24 h of skin wounding, the extent of EGFPPMN infiltration was not significantly different between three experimental groups (Figure 1A). However, between days 1 and 7, mice that received the high inoculum of S. aureus exhibited twice the number of PMNs in wounds, nearly a log higher bacterial burden, and wounds took more than 4 days longer to heal than mice inoculated with the low inoculum or control wounded mice treated with saline alone (Figure 1, A-F). These data show that PMN infiltrate the wound bed most rapidly over the first 24 hours following a skin wound and that the extent and persistence of phagocyte recruitment and wound resolution corresponds to the intensity of S. aureus infection. Since the major source of PMNs released into peripheral circulation is from the bone marrow compartment, we quantified the kinetics of EGFP-PMNs leaving the bone marrow and entering the venous blood compartment from mice inoculated with 1x107 CFUs of S. aureus as compared to uninfected wounds. Neutrophils were identified by gating on EGFPhigh, Gr-1high, and CD11bhigh fluorescence detected by flow cytometry. 10 The vast majority of EGFPhigh cells (e.g. ~95%) were Gr-1+ and CD11b+ confirming they were mature PMNs (Figure S1). By day 1, mice with a high S. aureus inoculum exhibited an 80% reduction in PMNs in bone marrow that was accompanied by a 2-fold increase in the blood that peaked at day 5 (Figure 2 and Table S1). The PMN counts returned to baseline levels in the bone marrow between days 3 to 5. In contrast, there were no significant changes in PMN cell numbers in bone marrow and blood from saline treated mice. Taken together, these findings indicate that S. aureus-infection elicits additional mobilization of PMNs from the bone marrow compartment to the circulation within 24 h of infection and correlates with increased numbers in the wound at later time points. Early recruitment and prolonged survival of PMNs are essential for S. aureus clearance and wound closure. To determine the influence of PMN recruitment and survival within the wound bed on bacterial clearance and wound resolution, mice were systemically depleted of PMNs with an anti-PMN monoclonal antibody (Gr-1) that was administered beginning 24 h prior or post wounding and S. aureus inoculation (SA-pre-Gr-1 and SA-post-Gr-1, respectively) (Figure 3A). SA-pre-Gr-1 mice showed a marked decrease in the initial recruitment of EGFP-PMNs to the infected-skin wounds, a sustained increase in bacterial burden, and impaired wound closure compared with mice that exhibited normal PMN trafficking (SA-isotype control) (Figure 3B-D). These results are consistent with the defective S. aureus clearance and larger skin lesions previously reported for mice depleted of PMNs 1, or with impaired PMN recruitment 13 . In contrast, SA-post Gr-1 11 mice had equal numbers of EGFP-PMNs recruited on day 1 as mice treated with SAisotype control (Figure 3B). Remarkably, the number of PMNs recruited by day 1 in the SA-post Gr-1 mice appeared to remain constant through day 8 and this was sufficient to control the S. aureus infection to a similar extent as the SA-isotype control that exhibited a ~100% boost in PMN infiltration. Furthermore, wound closure in the SA-post Gr-1 mice was actually accelerated compared with mice that had no depletion (SA-isotype), or delayed recruitment (SA-pre Gr-1) of PMNs (Figure 3D). These data indicate that the exuberant PMN recruitment induced by the high S. aureus inoculants actually delayed wound healing, and in fact the number of PMNs recruited during the initial 24 h of the inflammatory phase and maintained in the wound thereafter are sufficient to resolve infection. Mature neutrophils persist within S. aureus-infected wounds. Given the crucial role of PMN number and persistence for controlling the bacterial infection and wound resolution, the mechanism for PMN survival in the abscess was investigated. To determine the capacity of PMNs infiltrating the wound to evade apoptosis and survive in response to injury and infection, mature PMNs defined as EGFPhigh, c-kit-, Gr-1high and CD11bhigh cells were isolated from bone marrow cells by FACS-sorting (Figure 4A). These isolated cells were morphologically confirmed to be mature EGFP-PMNs and functionally confirmed to be non-proliferative (Figure S2A), which is consistent with previous reports 25 . A bolus of sorted PMNs were adoptively transferred by intravenous injection into C57BL/6 mice with S. aureus-infected or saline treated wounds. In both groups, the infused EGFP-PMNs were not detected in the 12 circulation after day 3 post-transfer (Figure S2B) and PMN fluorescence increased equivalently up to day 3 at which point S. aureus wounds exhibited sustained survival of PMN as compared to the saline treated wounds, which rapidly declined to baseline by day 8 (Figure 4B). Estimation of the half-life of EGFP-PMNs based on the rate of fluorescence signal decay after reaching their peak values (see Figure S2C) revealed that PMNs within S. aureus infected wounds exhibited nearly three-fold longer survival compared with saline treatment (Figure 4C; 4.96±0.38 days for S. aureus vs. 1.58±0.31 days for uninfected wounds; p<0.01). However, this prolonged survival was insufficient to account for the relatively constant EGFP-PMN numbers maintained up to day 8 in the wounds of mice treated with Gr-1 after infection (Figure 3B). Since additional PMN influx from the systemic compartment was blocked in presence of Gr-1, we hypothesized that a dynamic balance between PMN survival, apoptosis, and local production maintained this equilibrium in the EGFP signal. Bone marrow-derived c-kit+ progenitor cells proliferate and mobilize to the site of infection and locally differentiate into mature EGFP-PMNs. We next set out to determine whether Lin- c-kit+ cells are recruited from the circulation to the wound and contribute to the increased PMN numbers over the duration of infection. A 4-fold expansion of Lin- c-kit+ cells was detected in the bone marrow of S. aureus infected mice between days 1 and 3, which significantly exceeded the increase in mice with saline treated wounds (Figure S3). A thousand fold fewer Lin- c-kit+ cells were detected in blood compared to bone marrow and the number was not different 13 between saline treated and S. aureus infected mice, suggesting that they efficiently home to the wound and in greater numbers in response to infection. To directly image progenitor cell recruitment to the site of infection, EGFP - c-kit+ cells were FACS-sorted from bone marrow of EGFP-lys-mice, fluorescently labeled with Qtracker 705, and then injected via the tail vein into C57BL/6 mice (Figure 5A). There was equivalent efficiency of c-kit+ cell recruitment to S. aureus-infected or uninfected wounds at 24 h (Figure 5B). EGFP-PMN numbers in S. aureus-infected wounds steadily increased following infusion, reaching a level 30-fold greater than saline control wounds in which the signal decayed to baseline by day 8 (Figure 5C). It has previously been reported that Lin- c-kit+ cells of EGFP-lys-mice emit green fluorescence following myeloid lineage commitment in the bone marrow24. Thus, by sorting Lin- c-kit+ progenitors from EGFP-lys-mice bone marrow and infusing into C57BL/6 wild type mice, c-kit+ progenitors were shown to home from the circulation to the wound bed and effectively differentiate along the myeloid lineage into mature PMNs in the presence of S. aureus infection. To ensure that the EGFP-PMN signal was not due to myeloid progenitors that expanded in other peripheral tissue and subsequently emigrated to the wound, PMNs were depleted from the circulation by injection of Gr-1 mAb at days 1, 3, and 5 post-wounding. This treatment effectively eliminated EGFP-PMN detected in the blood (Figure S4A), but did not alter the functional capacity of Lin- c-kit+ cells to proliferate into EGFP-PMNs (Figure S4B). Thus, we conclude that the further rise in EGFP-PMN signal detected in S. aureus-infected wounds was derived from differentiation of c-kit+ progenitors in the wound. 14 Myeloid progenitors isolated from infected wounds differentiate into mature PMNs. Experiments were performed to further confirm that Lin- c-kit+ progenitors that home to S. aureus-infected wounds can differentiate into mature EGFP-PMNs. Cells were harvested on day 3 from infected and uninfected wounds of EGFP-lys-mice and cultured in an ex vivo colony forming unit (CFU) assay. Infected wounds exhibited a 3fold increase in both the total number of colonies and EGFP expressing granulocytic colonies compared with uninfected wounds (Figure 5D). A cytospin of cells extracted from the CFU assay confirmed that the majority of cells were mature PMNs (Figure 5E). As a control, EGFP-PMN isolated from blood and cultured in the CFU assay showed no expansion potential and fluorescence decayed to background within 3 days (data not shown). These data confirm that Lin- c-kit+ progenitors receive an early signal at the site of S. aureus abscess to proliferate and differentiate into mature PMNs as early as day 3 following infection. Depletion of c-kit+ progenitors diminishes PMN expansion and mice survival compared with PMN depletion alone. A final set of studies were carried out to reveal the functional significance of ckit+ myeloid progenitor trafficking from the bone marrow to the wound for sustaining PMN numbers and resolution of S. aureus infection. Antibody to c-kit+ cells denoted ACK2 was injected 5 days before and 1 day following wounding and infection (Figure 6A). This duration of pretreatment effectively depleted c-kit+ progenitor cells in the bone marrow by more than ~60%, while PMN numbers decreased by only ~30% compared with injection of an isotype control antibody (Figure S5). Mice were simultaneously 15 pretreated with ACK2 and Gr-1 in order to block the trafficking of both c-kit+ progenitors and mature PMNs. This resulted in a 3-fold decrease in the EGFP-PMN signal at day 1 and a significantly diminished rate of PMN proliferation over time compared to depletion of PMNs alone (Figure 6B). We confirmed that cells recovered from wounds of SA-pre Gr-1 mice exhibited the capacity to proliferate into EGFP-PMN colonies ex vivo in the Methocult culture assay (Figure 6C). Blocking c-kit+ trafficking in the ACK2-treated mice resulted in a diminished rate of survival of infected mice, which dropped from 80% to 40% by day 2 for SA-pre ACK2-Gr-1 mice compared to SA-pre Gr-1 mice (Figure 6D). These data provide a direct demonstration of the importance of myeloid progenitor trafficking and local expansion within the site of infection to provide adequate numbers of PMN to prevent dissemination of the bacteria and host survival. Discussion Neutrophils are rapidly mobilized from the bone marrow in sufficient numbers to provide an effective innate immune response against an invading pathogen such as S. aureus1,13,26. However, the mechanisms by which PMNs are recruited and maintain an abscess to control the infection and promote bacterial clearance, while promoting wound resolution are not completely defined. Here, we applied non-invasive in vivo fluorescence imaging of genetically EGFP-tagged PMNs to provide a quantitative measure of the number present in the wound over time and as a function of S. aureus burden. Using this model, we identified three distinct mechanisms that act in concert to maintain sufficient numbers of PMNs for host defense in S. aureus-infected wounds, including: (1) a robust and sustained mobilization of PMNs from the bone marrow that correlated with infection 16 burden, (2) a prolonged in vivo survival of PMNs within the abscess, and (3) trafficking of c-kit+ progenitor cells that proliferate and differentiate at the site of the abscess in the skin to produce mature PMNs (Figure 7A). These data demonstrate that S. aureusinfected wounds provide important signals that prolong PMN lifespan, and remarkably the proliferation and differentiation of mature PMNs from bone marrow-derived c-kit+ progenitor cells that have emigrated to the site of infection. Host-pathogen interactions are initiated through molecular sensing of conserved pathogen-associated molecular patterns (PAMPs) of microbes by pattern recognition receptors (PRRs), such as TLRs, which are expressed on many different types of cells, including innate immune cells (e.g. macrophages and dendritic cells) and epithelial cells27. During the inflammatory phase of the innate immune response this pathway triggers the release of pro-inflammatory mediators such as IL-17, IL-1, TNF-, and IFN- which lead to production of chemokines (KC and MIP- 2) that trigger recruitment of circulating PMNs to the site of infection11,13,26,28. In addition, hematopoietic factors (e.g. G-CSF and GM-CSF) are also produced, which promote granulopoiesis in the bone marrow29. Our data demonstrate that, during the initial 24 h after skin wounding, signals are released from the wound to elicit a marked increase in PMN recruitment, which is significantly augmented in the S. aureus-infected wounds by 48 h. This was accompanied by a concomitant 80% reduction in the bone marrow PMN count and a comparable rise in circulating PMNs that was sustained up to day 5. This is in contrast to the negligible change in PMNs in aseptic wounds, which did not increase in number after 24 h. These data demonstrate that the presence of the S. aureus infection promotes an increase in 17 PMN expansion and trafficking from the bone marrow to a cutaneous wound to control the infection. To understand the contribution of PMNs entering the site of infection from the circulation versus local PMNs that are maintained in the abscess, we depleted PMNs in the circulation with an anti-Gr-1 mAb administered either 24 h before or after wounding and infection. Immunodepletion before wounding and infection revealed an essential role for the rapid influx of PMN from the circulation for both controlling S. aureus bacterial burden and wound resolution. In contrast, immunodepletion at 24 h after wounding and infection provided clear evidence that PMN numbers are maintained beyond 8 days in an abscess and importantly these are necessary for resolving the infection. Adoptive transfer of FACS-sorted EGFP-PMNs in wild-type mice demonstrated an equivalent efficiency of recruitment between S. aureus-infected and uninfected wounds. The kinetics of the appearance of infused EGFP-PMN in the wounds of C57BL/6 wild type mice were similar to those in the EGFP-lys-mice, thus revealing that the early inflammatory phase provides potent innate signaling that is independent of PAMPs. In contrast, PMNs recruited to infected wounds exhibited a tripling in lifespan, and this population, without further PMN recruitment from the bone marrow was sufficient to control infection. In fact, limiting the numbers of PMNs recruited to the S. aureus-infected was not only sufficient in clearing the bacteria, but allowed the wound to resolve more rapidly than normal control mice exhibiting exuberant PMN recruitment. These data provide evidence that increased PMNs numbers do not necessarily have an immune benefit and may in fact be detrimental to wound healing. Furthermore, a PMN-rich environment has previously been shown to potentiate S. aureus pathogenesis by facilitating bacterial survival within 18 the PMNs themselves26. Therefore, a therapeutic advantage against infected wounds may include immunomodulatory strategies aimed at optimizing the numbers of PMNs to clear the infection while minimizing the PMN-rich environment and unwanted PMN-induced inflammation that inhibits wound healing. In the present study, we demonstrate that S. aureus infection effectively extends the half-life of PMNs within the wounds by three-fold. Applying a simple mathematical model to predict the dynamics of three separate populations, we estimate that prolonged survival of PMN recruited from the inflammatory phase (Psurvival) make up 60% of total numbers at day 2 and diminish to ~20% by day 5 (Figure 7B and Figure S6). The remaining 40% are equally divided between PMN recruited from the systemic compartment (Pinflux) and those differentiated from the c-kit+ myeloid progenitors within the wound (Pckit). While the rate of c-kit+ progenitor proliferation out to day 5 provides a relatively constant source of PMN, the robust rate of PMN influx from peripheral blood appears to balance the loss due to clearance and apoptosis. The extended half-life of PMNs is presumably mediated by anti-apoptotic signals that are generated during an infection30. Some of these anti-apoptotic signals have been described in vitro, including pro-inflammatory cytokines (e.g. IL-6, IFN-, G-CSF, GM-CSF)31 and bacterial products (e.g. LPS and lipoteichoic acid)17. A recent report demonstrated that IL-2-activated NK cells produce chemokines that prolong PMN survival and boost their phagocytic and oxidative microbicidal capacity32. Other in vitro studies have demonstrated that the concentration of the inocula can directly influence PMN apoptosis. For example, human PMNs undergo rapid apoptosis under culture conditions with a high ratio of bacteria (S. aureus or E. coli) to PMNs (100:1), in contrast to delayed apoptosis at a low ratio (1:1)19. 19 Furthermore, in vivo studies have also demonstrated that the concentration of the inoculum can influence PMN survival in a similar manner to our results with S. aureusinfected skin wounds. Mice challenged in vivo with a lethal inoculum of L. monocytogenes had rapid PMN apoptosis via a TLR2-dependent mechanism33. In contrast, infection with lower sub-lethal inocula of L. monocytogenes resulted in robust PMN trafficking to infected tissue, which was associated with successful bacterial clearance. Collectively, our findings are consistent with these previous studies demonstrating that the bacterial burden plays a key role in PMN survival and extent of influx, which is likely triggered by anti-apoptotic signals generated during the infection and local production of chemokines. We demonstrated that adoptive transfer of Lin- c-kit+ progenitor cells home to the site of S. aureus infection where they expanded the population of mature PMNs. Evidence supporting this were that depletion of c-kit+ cells with ACK2 antibody nearly abolished the increase in wound PMNs and the demonstration that cells recovered from S. aureus infected wounds contained myeloid progenitor cells that had the potential to give rise to mature PMNs. We conclude that bone marrow-derived c-kit+ progenitor cells traffic to sites of robust inflammation and infection and directly participate in PMN abscess formation. Based on antibody blocking studies and a mathematical model, we predict that c-kit+ progenitor cells home from the bone marrow early during the inflammatory phase and sustain PMN numbers during abscess formation. Although the precise mechanism by which S. aureus induced local c-kit+ progenitor cell proliferation and differentiation into functional PMNs in infected wounds was not directly assessed, previous reports suggest that their proliferation was induced by 20 either direct interactions between bacteria and c-kit+ progenitors or indirectly by hematopoietic cytokines. For example, Lin- c-kit+ progenitor cells express TLRs on their surface and the direct binding of bacterial proteins to Lin- c-kit+ cells can trigger rapid myeloid differentiation in vitro23. Another study demonstrated that TLRs can induce Linc-kit+ cell to expand into a dendritic cell phenotype, yet the proliferative potential for PMN expansion and their functional role during the progression of bacterial infection was not evaluated22. To provide insight into the molecular mechanisms underlying the differentiation and expansion of the progenitor cells in the infected wounds, we evaluated the potential role of TLRs in inducing Lin- c-kit+ cell expansion from mice deficient in MyD88, an adaptor protein central to signaling via the TLR2 and IL-1 receptor pathways34. We found that c-kit+ progenitors isolated from the bone marrow of MyD88 knockout mice formed two-fold fewer granulocytic colonies than wild type in the presence of heat-killed S. aureus in the ex vivo culture assay (Figure S7). These results point to several pathways by which S. aureus may promote c-kit+ progenitor activation. Within the wound S. aureus sheds membrane lipoproteins that, once bound by TLR2, activate MyD88 that in turn signals transcription factor NF-κB activity in target cells. Since Lin- c-kit+ progenitor cells express TLRs on their surface, one mechanism may involve the direct binding of bacterial lipoproteins to Lin- c-kit+ cells to trigger rapid myeloid differentiation23 (Figure 7A). Another mechanism may involve TLR induced Lin- c-kit+ cell differentiation into a dendritic cell phenotype, as recently reported22. We are currently engaged in studies to determine the differential contribution of TLR and IL1R ligands and how they may directly or indirectly elicit differentiation and proliferation of c-kit+ progenitors in the wound. 21 An alternate mechanism supporting PMN expansion is via production of G-CSF that is significantly increased during bacterial infection30,35 and can specifically direct the proliferation and differentiation of bone marrow granulocyte-macrophage progenitors (GMPs) into granulocytes in vitro36. Future study will be directed toward understanding the mechanisms by which S. aureus infection promotes c-kit+ progenitor cell emigration from the bone marrow into the circulation and subsequently into a wound, and the generation of the local signals within the abscess that elicits myeloid progenitor expansion. In summary, these studies demonstrate that in a S. aureus-infected wound, the formation and maintenance of the PMN abscess involves not only continual recruitment of PMNs from the circulation and bone marrow, but also prolonged PMN survival in combination with local c-kit+ progenitor cell proliferation and differentiation into mature PMNs at the site of infection. These findings represent an important demonstration of infection-induced PMN survival in vivo and a pivotal role for myeloid progenitor cells in the resolution of a PMN abscess. From a clinical point of view, the identification of the mechanisms that govern PMN abscess formation raises the possibility that PMN and ckit+ progenitor cell recruitment, circulating numbers, or activation state could be modulated so that they are sufficient to clear a microbial infection while preventing aberrant inflammation that could exacerbate the infection and delay wound healing. 22 Acknowledgments We thank Dr. Thomas Graf for generously providing the EGFP-lysozyme transgenic mice. This work was supported by grants National Institute of Health (NIH) AI42794 (to S.I.S). L.S.M was supported by NIH AI078910 and AR054534. J.L.G was supported by Floyd and Mary Schwall Fellowship in Biomedical Research from UC Davis. Authorship Contribution: M.K and J.L.G. designed experiments and performed the research, collected and analyzed data, and wrote the manuscript; C.K. and N.W. assisted with the experiments; D.L.B. and F.E.C. designed experiments and reviewed the manuscript. L.S.M designed the research and wrote the manuscript; S.I.S designed the experiments, interpreted the data, and wrote the manuscript. Conflict-of-interest disclosure: The authors declare no competing financial interests. 23 References 1. Molne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun. 2000;68(11):6162-6167. 2. Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65(7):2517-2521. 3. Corbin BD, Seeley EH, Raab A, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319(5865):962-965. 4. Kim MH, Liu W, Borjesson DL, et al. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol. 2008;128(7):1812-1820. 5. Li Y, Karlin A, Loike JD, Silverstein SC. A critical concentration of neutrophils is required for effective bacterial killing in suspension. Proc Natl Acad Sci U S A. 2002;99(12):8289-8294. 6. Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology. 2008;125(3):281-288. 7. Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846-852. 8. Armstrong DA, Major TA, Chudyk A, Hamilton TA. Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J Leukoc Biol. 2004;75641-648. 9. De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol. 2008;180(6):4308-4315. 10. Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol. 2006;26(4):307-316. 11. Cho JS, Pietras EM, Garcia NC, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120(5):17621773. 12. Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):273-279. 13. Miller LS, O'Connell RM, Gutierrez MA, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24(1):79-91. 14. Miller LS, Pietras EM, Uricchio LH, et al. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179(10):6933-6942. 15. Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100(3):854-861. 16. Cheretakis C, Leung R, Sun CX, Dror Y, Glogauer M. Timing of neutrophil tissue repopulation predicts restoration of innate immune protection in a murine bone marrow transplantation model. Blood. 2006;108(8):2821-2826. 17. Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80(8):2012-2020. 24 18. Garlichs CD, Eskafi S, Cicha I, et al. Delay of neutrophil apoptosis in acute coronary syndromes. J Leukoc Biol. 2004;75(5):828-835. 19. Ocana MG, Asensi V, Montes AH, Meana A, Celada A, Valle-Garay E. Autoregulation mechanism of human neutrophil apoptosis during bacterial infection. Mol Immunol. 2008;45(7):2087-2096. 20. Yamada M, Kubo H, Kobayashi S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004;172(2):1266-1272. 21. Si Y, Tsou CL, Croft K, Charo IF. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest. 2010;120(4):1192-1203. 22. Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131(5):994-1008. 23. Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24(6):801-812. 24. Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96(2):719-726. 25. Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201(11):1771-1780. 26. McLoughlin RM, Lee JC, Kasper DL, Tzianabos AO. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J Immunol. 2008;181(2):1323-1332. 27. Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163(1):1-5. 28. Zhang Y, Ramos BF, Jakschik BA. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992;258(5090):19571959. 29. Zhang P, Quinton LJ, Gamble L, Bagby GJ, Summer WR, Nelson S. The granulopoietic cytokine response and enhancement of granulopoiesis in mice during endotoxemia. Shock. 2005;23(4):344-352. 30. Saba S, Soong G, Greenberg S, Prince A. Bacterial stimulation of epithelial GCSF and GM-CSF expression promotes PMN survival in CF airways. Am J Respir Cell Mol Biol. 2002;27(5):561-567. 31. Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43(1-3):25-61. 32. Bhatnagar N, Hong HS, Krishnaswamy JK, et al. Cytokine-activated NK cells inhibit PMN apoptosis and preserve their functional capacity. Blood. 2010;116(8):13081316. 33. Navarini AA, Lang KS, Verschoor A, et al. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc Natl Acad Sci U S A. 2009;106(17):7107-7112. 25 34. Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci. 2002;27(9):474-482. 35. Watari K, Asano S, Shirafuji N, et al. Serum granulocyte colony-stimulating factor levels in healthy volunteers and patients with various disorders as estimated by enzyme immunoassay. Blood. 1989;73(1):117-122. 36. Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325(5937):217-218. 26 Figure Legends Figure 1. Effects of S. aureus inoculum on PMN trafficking and tissue healing. EGFP-lys-Mice were inoculated with saline or low dose S. aureus (2x106 CFU, SA-2e6 CFU), or sub-lethal high dose S. aureus (1x107 CFU, SA-1e7 CFU) on back skin wounds, and kinetics of wound EGFP-PMN fluorescence (A-B), S. aureus (SA) bioluminescence (C-D), and wound closure (E-F) were determined. Depicted are representative images of wound EGFP-PMN (B), bioluminescent S. aureus (D), and wound size (F). Data are derived from six to eight mice in each group and expressed as mean ± SEM. *, p<0.05 vs Saline group and #, p<0.05 vs SA-2e6 CFU group. Figure 2. Bone marrow mobilization of mature PMNs into peripheral blood circulation in response to S. aureus wound infection. Mature EGFP-PMNs were gated on EGFPhigh Gr-1high cells. Kinetics of EGFPhigh Gr-1high cells in the bone marrow (A) and peripheral blood (B) from mice treated with either sterile saline or S. aureus (1x107 CFU). Data are derived from three to four mice in each group and expressed as mean ± SEM. *, p<0.05 and **, p<0.01 vs Saline group. Figure 3. Effects of time dependent immunodepletion of systemic PMNs on S. aureus wound infection. (A) Experimental design for early and delayed depletion of systemic EGFP-PMNs. Systemic PMNs were depleted with anti-neutrophil antibody treatment beginning either 24 h before (pre Gr-1) or 24 h after (post Gr-1) onset of the infection (1x107 CFU S. aureus) and then every two days up to day 5. Kinetics of wound EGFPPMNs (B), S. aureus burden (C), and wound closure (D). Data are derived from five to 27 six mice in each group and expressed as mean ± SEM. *, p<0.05 vs SA-Isotype control group. Figure 4. Adoptive transfer of PMNs and recruitment to S. aureus infected wounds. (A) FACS-sorting of mature EGFP-PMNs (c-kit- EGFPhigh Gr-1high CD11bhigh cells) and cytospin image. In order to avoid any inclusion of cells with proliferative capacity, c-kit+ progenitor cells were gated out. The FACS sorted cells were intravenously transferred (5x106 cells in 0.1 mL sterile saline) to C57BL/6 mice whose wounds were treated with either sterile saline or S. aureus (1x107 CFU) and kinetics of EGFP-PMNs within the wound were determined (B). (C) The half-life of wound EGFP-PMNs was quantified from the EGFP-PMN kinetic data using equations described in Methods. Data are derived from four mice in each group and expressed as mean ± SEM. *, p<0.01 vs Saline control group. Figure 5. Bone marrow-derived c-kit+ progenitors recruit to wounds and give rise to EGFP-PMN in the presence of S. aureus. (A-C) Adoptive transfer of EGFP- c-kit+ cells from EGFP-lys-mice into C57BL/6 wild-type mice. (A) FACS sorted EGFP- c-kit+ cells were labeled with Qtracker 705 and intravenously transferred (5x105 cells in 0.1 mL sterile saline) to C57BL/6 mice whose wounds were treated with either sterile saline or S. aureus (1x107 CFU). (B) Emigrated Qtracker 705 labeled-EGFP- c-kit+ cells were detected at 24 h post-transfer in wounds. n=2 mice in each group. (C) Kinetics of EGFPPMNs within the S. aureus infected wound following adoptive transfer of EGFP- c-kit+ cells. *, p<0.05 vs Saline-Gr-1 group. Data derived from three to four mice in each group. 28 (D and E) Ex-vivo G-CFU assay of wound cells harvested from EGFP-lys-mice treated with saline or S. aureus (1x107 CFU). (D) Three days after wounding, S. aureus (1x107 CFU) or saline treated skin wounds were collected from EGFP-lys-mice, digested and plated in methylcellulose medium. Data are from three separate experiments and represents mean colony counts from S. aureus (n=5 mice) and saline (n=3 mice) treated wounds analyzed 7 days after plating. *, p<0.05 vs Saline group. CFU=colony forming unit; GM/GEMM=granulocyte-macrophage/granulocyte, erythroid, megakaryocyte, macrophage; G=granulocyte; M=monocyte; E-BFU=erythroid blast forming unit. (E) Clockwise from top left, EGFP+ colony from S. aureus infected wound, cytospin of cells extracted from EGFP+ G-CFU colony, and representative negative control non-EGFP colony. CFU images are overlays of phase contrast and fluorescent images taken with 20X objective (bar represents 50 μm). Cytospin image taken with 50X objective (bar represents 10 μm). Data are expressed as mean ± SEM. Figure 6. C-kit+ progenitor proliferation within wounds contributes to immune protection against S. aureus infection. (A) Experimental design for immunodepletion of c-kit+ cells and PMNs prior to S. aureus infection (1x107 CFU) and skin wounding (SApre ACK2-Gr-1). (B) Comparison of kinetics of wound EGFP-PMNs in S. aureus infected EGFP-lys-mice with SA-pre ACK2-Gr-1 and SA-pre Gr-1 treatment (refer to the Figure 3A for experimental design of SA-pre Gr-1 treatment). (C) EGFP+ colony derived from cells harvested from EGFP-lys-mice with SA-pre Gr-1 treatment. At day 5 postinfection, wounded and S. aureus-infected skin biopsies were collected from EGFP-lysmice with SA-pre Gr-1 treatment, digested and plated in methylcellulose medium. 29 Representative image of EGFP+ colony 7 days after plating. (D) Comparison of survival rate between EGFP-lys-mice with SA-pre ACK2-Gr-1 and SA-pre Gr-1 treatment. *; p<0.05 vs SA-pre Gr-1. Figure 7. Dynamic contributions of three distinct populations of PMN constitute the innate immune response to S. aureus infection. (A) A model depicting the dynamic feedback between bone marrow compartment and wound compartment during bacterial infection. Local S. aureus infection triggers signals (e.g. hematopoietic cytokines) to maintain an enhanced number of PMNs in infected tissue. This can be largely achieved by increased production and mobilization of PMNs in the bone marrow and their recruitment into local infected tissue, where their lifespan is markedly extended to provide sustained immune protection. Additionally, bone marrow c-kit+ progenitor expansion and mobilization are activated by S. Aureus. Progenitor cells infiltrate the site of infection where they proliferate and differentiate into functional PMNs, in part via a MyD88-dependent mechanism to provide additional immune protection. Pinflux represents number of newly infiltrated PMNs from blood to infected tissue during S. aureus infection; Pckit represents the number of newly produced PMNs in the tissue by proliferation of c-kit progenitors; Psurvival represents the number of early recruited PMNs that are persisted after day 1 of infection within wound. (B) Relative contribution of enhanced PMN survival and their local production by tissue infiltrated c-kit+ cells which contribute in equal proportion by day 3 to maintain tissue PMN number during S. aureus infection. Mathematical modeling predicts the relative 30 contributions of each compartment between days 1 and 5 of S. aureus infection (1x107 CFU) (see Supplemental Methods in detail). 31 Figure 1 32 Figure 2 33 Figure 3 34 Figure 4 35 Figure 5 36 Figure 6 37 Figure 7 Supplemental Methods Mouse model of S. aureus wound infection Mice were anesthetized with ketamine/xylazine (i.p., 80/10 mg/kg, Sigma, MO). Back skin hair was shaved, and then antiseptically prepared with 10% w/v povidine-iodine and 70% ethanol. A circular full thickness wound (6mm in diameter) was made using a skin biopsy punch (Robbins instruments Inc., NJ). Immediately after skin wounding, mice were inoculated locally with sterile saline or low (2x106 CFU) and sub-lethal high dose (1x107 CFU) of a bioluminescent strain of S. aureus at sites of skin wound. Non-invasive quantification of wound EGFP-PMNs trafficking The trafficking of EGFP-PMNs at wound sites over time was determined non-invasively. The in vivo imaging of EGFP-PMN fluorescence appearing at the site of the back skin wounds was performed using a whole-body small animal fluorescence imaging system (Xenogen IVIS 100 system, Xenogen Inc., CA). Mice were placed into the imaging chamber of the system after being anesthetized by isoflurane gas (2%, inhalation). The EGFP-expressing neutrophils within wound area were visualized using a GFP filter (excitation at 445~490 nm and emission at 515~575 nm) at an exposure time of 1 second. Analysis of the images were performed using Live Image Pro. 2.5 software (Caliper Life Science, CA) and fluorescence intensities expressed as average radiance (photons per cm2 per steradian) were measured by drawing a circular region of interest (ROI) over the entire wound area. Preparation and in vivo bioluminescent imaging of S. aureus Bioluminescent strain (SH1000) of S. aureus was streaked onto Tryptic soy agar (Tryptic soy broth [TSB] + 1.5% Bacto Agar). Colonies of S. aureus were grown overnight at 37°C in a shaking incubator (240 rpm) in TSB. Midlogarithmic phase bacteria were obtained after a 3 hr subculture of 1:100 dilution of the overnight culture and cultures were performed in the presence of the selection agent chloramphenicol (10 μg/ml). Bacterial cells were pelleted, resuspended, and washed three times in PBS. Bacterial concentrations were estimated with a spectrophotometer by determining the absorbance at 600 nm (A600). Colony-forming units (CFUs) were verified by plating dilutions of the inoculum onto TSB agar ± chloramphenicol overnight. Then 100 μl of midlogarithmic growth phase S. aureus (2x106 CFU/100mL or 1x107 CFU/100mL) was inoculated into the wounded sites of the mice. Dynamic changes in actively metabolizing S. aureus within a wound area were visualized using the Xenogen imaging system at an exposure time of 1 min, which enabled a non-invasive detection of S. aureus bioluminescence. Wound S. aureus burden was determined by quantifying total flux (photons per second) within a circular region of interest (ROI) drawn over the entire wound area. Flow cytometric immunophenotyping assay Bone marrow and blood cells were collected from EGFP-lys-mice and adjusted to 1 x 106 cells/mL. The following antibodies were used for immunophenotyping: Phycoerythrin (PE) conjugated anti-mouse Gr-1 and PE-Cy5 conjugated anti-mouse CD11b (all from eBioscience, CA); APC conjugated anti-mouse CD117 (c-kit) antibody and PE conjugated lineage markers (Gr-1 to detect granulocytes, TER-119 to detect proerythroblasts to mature erythrocytes, B220 and CD3 to detect B and T lymphocytes respectively) (all from BD Pharmingen, CA). Events were acquired using a FACScan flow cytometer (Becton Dickinson, NJ). Data was analyzed using FlowJo software (Treestar, Inc., OR). FACS cell sorting Bone marrow cells isolated from EGFP-lys-mice were sorted into c-kit+ progenitors and mature EGFP-PMNs using the MoFlo cell sorter (Beckman Coulter, Inc., CA). For sorting of c-kit+ progenitor cells, bone marrow cells were incubated with APC conjugated anti-mouse CD117 antibody (R&D Systems, MN) and sorted into EGFP- c-kit+ cells. For sorting of non-proliferative mature EGFP-PMNs, bone marrow cells were incubated with APC conjugated anti-mouse CD117 antibody (R&D Systems, MN), PE conjugated anti-mouse Gr-1, and PE-Cy5 conjugated anti-mouse CD11b (all from eBioscience, CA) and sorted into c-kit- EGFPhigh Gr-1high CD11bhigh cells. Tissue half-life of PMNs Neutrophil tissue half-life was determined by quantifying the rate of PMN clearance from sites of wound infection. FACS sorted c-kit- EGFPhigh Gr-1high CD11bhigh mature EGFP-PMNs (5x106 cells in 100 L sterile saline) were intravenously injected via tail-vein to C57BL/6 mice 3hr after wounding and S. aureus (1x107 CFU) (or sterile saline) treatment on wounded skin and then fluorescence of EGFP-PMNs was measured at wound sites using the Xenogen imaging system as described above. PMN half-life (t1/2) was calculated as previously described using the following equation 1: Nt=No e-k(t-) for t≥ and t1/2=ln2/k, where Nt is the amount of EGFP-PMNs in the tissue at time t, and No is the number of EGFP-PMNs at time at which their levels reaches peak value. k is the rate constant of PMN disappearance from the wound due to either apoptosis or clearance by macrophages and the value is determined by fitting the decay rate of EGFP-PMNs from S. aureus infected versus saline treated wounds following their adoptive transfer to recipient mice. Colony forming unit assay At selected time points post-wounding and saline or S. aureus (1x107 CFU) inoculation, either bone marrow cells or skin wounds were collected from EGFP-lys-mice. For collection of progenitor cells from skin wounds, 8 mm diameter tissue biopsies were coarsely chopped and placed into 5 mL of RMPI 1640 (Invitrogen, CA) supplemented with 10 mM HEPES, 1% penicillin-streptomycin, 1% collagenase, 0.025% DNase I (all from Sigma-Aldrich, MO), and 1% FBS and incubated, rocking, for 1 hr at 37⁰C. After incubation, the tissue digest was washed with cold 5 mM EDTA in PBS and passed consecutively through 70 m and 35 m filters. Cells were pelleted at 1000 g for 12 minutes. C-kit+ progenitors were enriched by positive selection using CD117 magnetic bead separation (Miltenyi Biotec Inc., CA) and resuspended in RMPI 1640 and plated in duplicate in methylcellulose media supplemented with cytokines to support growth of myeloid progenitors (Methocult GF M3434, Stem Cell Tech, Vancouver, Canada) at a cell density of 3x105/mL. Colony forming units were quantified 7 days after plating using a Leica DMIL inverted microscope (Leica Microsystems, IL). The presence of EGFP positive colonies was assessed using a Leica DMI6000B fluorescent microscope and images were captured using Image Pro 6.3 software (Media Cybernetics, MD). Mathematical modeling to estimate tissue PMN number during S. aureus infection A mathematical model was developed to predict the kinetics of PMN influx, survival, and production as observed by whole animal fluorescence imaging during the course of S. aureus infection (1x107 CFU). We limited the modeling to estimate PMN numbers during the time course between days 1 and 5 for three reasons. First, our experimental data indicates that PMN recruitment during the initial 24hr was not significantly influenced by S. aureus infection, however, striking differences occurred from days 1 through 5 (Figure 1A and Figure 3B). The rapid increase suggests the effects of S. aureus infection on tissue PMNs trafficking is prominent during this period. Second, immunodepletion of blood PMNs with repetitive anti-Gr-1 antibody treatment effectively inhibited new PMN recruitment from blood to wound up to day 5 of wounding and infection (Figure 3B). Third, pretreatment with anti-c-kit ACK2 and Gr-1 antibodies effectively abolished local production and suppressed the increase in PMN signal during this period (Figure 6B). These antibody blocking studies provided kinetic data to delineate total PMN numbers within the wound (Pwound) as a function of the dynamics of three distinct populations; (1) continuous PMN infiltration from blood to infected tissue post-infection (Pinflux), (2) the survival of early recruited PMNs following day 1 of infection (Psurvival), and (3) PMN production in the tissue by proliferation of tissue infiltrated c-kit+ progenitors (Pckit). Pwound=Pinflux+Psurvival+Pckit (1) Based on previous studies of PMN dynamics2, we assume they are cleared exponentially within the wound over time and thus the continuous function Psurvival was assumed using an exponential decay function and defined at t > as follows, Psurvival=No e-k(t-) for t > (2) Where No is total number of PMNs within wounded skin and this boundary condition is evaluated at t==24 hr. The k is the rate constant of PMN disappearance from the wound due to either apoptosis or clearance by macrophages and the value is determined by fitting the decay rate of genetically labeled EGFP-PMNs from S. aureus infected versus saline treated wounds following their adoptive transfer to recipient mice (refer to Figure S2C). The continuous function Pckit is determined by mass balance under the condition in which PMN influx from blood compartment was completely blocked (SA-pre-Gr-1; Figure 3B). The rate of EGFP-PMN arising from c-kit+ myeloid cells was confirmed by blocking progenitor recruitment to wound with ACK2 in the presence of Gr-1 (Figure 6B). PMN production in the wound under conditions in which their recruitment from the systemic compartment is blocked (i.e. Pre-Gr-1) is maintained by a balance between c-kit+ myeloid proliferation and PMN clearance from the wound with a rate constant of k as follows (see Figure S6A), dPSA-pre-Gr-1/dt=-k PSA-pre-Gr-1+dPckit/dt (3) A continuous function of progenitor proliferation Pckit was assumed based on kinetics of Figure 6B which are solely due to c-kit+ proliferation as follows, Pckit= (1-e-t) (4) Where is the rate constant of proliferation and is the steady state value of PMN production by c-kit+ cell proliferation in infected wound. Inserting the time derivative of equation (4) into equation (3) and solving the first-order differential equation yields an analytical solution as follows, PSA-preGr-1=/(-k)*(e-kt -e-t) (5) The parameters and can be estimated by fitting equation (5) into SA-pre Gr-1 data in Figure 6B. The parameter estimate of best fit for Pre Gr-1 experimental data is shown in Figure S6B. The contribution of continuous PMN influx from systemic circulation to the wound compartment following day 1 of infection is determined by inserting estimated functions of Psurvival and Pckit from equations (2) and (4) into equation (1) as follows, Pinflux= Pwound - No e-k(t-) -(1-e-t) (6) Since Pwound is determined from experimental data of EGFP-PMN fluorescence signal in SA 1e7 CFU-IgG (data in Figure 3B), Pinflux is determined as Pinflux= PSA1e7CFU-IgG - No e-k(t-) -(1-e-t) (7) In vitro stimulation of c-kit+ bone marrow progenitor cells with heat-killed S. aureus Bone marrow cells from C57BL/6 and MyD88 knock-out mice with C57BL/6 background (a kind gift from Dr. Richard Flavell at UC Davis) were enriched for c-kit+ progenitors using CD117 magnetic beads (Miltenyi Biotec Inc., CA). Fifty-thousand cells per condition were incubated with heat-killed (95⁰C for 5 minutes) S. aureus or no stimulus for 48 h in 100 μl StemSpan media (Stem Cell Technologies, BC, Canada) along with the cytokines SCF (20 ng/ml) and Flt-3 ligand (100 ng/ml) (both from Peprotech, NJ) to promote cell viability. Cells were then collected and plated in Methocult GF M3434 (Stem Cell Technologies) at a cell density of 3x105/mL. Colony forming units were quantified 7 days after plating using a Leica DMIL inverted microscope (Leica Microsystems, IL). References 1. Cheretakis C, Leung R, Sun CX, Dror Y, Glogauer M. Timing of neutrophil tissue repopulation predicts restoration of innate immune protection in a murine bone marrow transplantation model. Blood. 2006;108:2821-2826. 2. Rosinski M, Yarmush ML, Berthiaume F. Quantitative dynamics of in vivo bone marrow neutrophil production and egress in response to injury and infection. Ann Biomed Eng. 2004;32:1108-1119. Supplemental Table Table S1. The dynamic changes of EGFP hi-Gr-1 hi cell populations in the bone marrow and blood from EGFPlys-mice treated with either saline or S. aureus (1x107 CFU) infection. Data are derived from three to four mice in each group and expressed as mean ± SEM. *, p<0.05 and **, p<0.01 vs Saline group. Bone marrow Treatment Total Number of bone marrow cells per femur EGFP hiGr-1 hi cell (% bone marrow cells) 32.2 ± 1.4 ( x 106 cells) 41.57 ± 3.28 Day 1 28.7 ± 0.6 ( x 106 cells) Total number of leukocytes per mL blood EGFP hiGr-1 hi cell (% blood leukocytes) EGFP hiGr-1 hi cell (# per mL blood) 13.4 ± 1.06 ( x 106 ells) 8.60 ± 0.78 ( x 106 cells) 5.61 ± 1.27 0.48 ± 0.10 ( x 106 ells) 34.37 ± 3.12 9.84 ± 0.88 ( x 106 ells) 6.15 ± 0.05 ( x 106 cells) 4.99 ± 0.39 0.31 ± 0.02 ( x 106 ells) Day 3 29.3 ± 1.8 ( x 106 cells) 33.13 ± 2.91 9.03 ± 0.86 ( x 106 ells) 6.50 ± 1.70 ( x 106 cells) 6.31 ± 1.27 0.48 ± 0.10 ( x 106 ells) Day 5 36.8 ± 0.3 ( x 106 cells) 36.42 ± 4.46 12.9 ± 1.65 ( x 106 ells) 4.55 ± 0.75 ( x 106 cells) 17.74 ± 3.83 0.89 ± 0.17 ( x 106 ells) Day 1 23.2 ± 0.1 ( x 106 cells) 9.66 ± 2.01** 2.24 ± 0.47** ( x 106 ells) 2.81 ±0.13 ( x 106 cells) 33.2 ± 7.63** 0.93 ± 0.20* ( x 106 ells) Day 3 23.2 ± 4.4 ( x 106 cells) 28.25 ± 2.07 6.03 ± 0.49* ( x 106 ells) 4.87 ± 0.74 ( x 106 cells) 15.28 ± 2.14* 0.65 ± 0.12 ( x 106 ells) Day 5 34.5 ± 2.0 ( x 106 cells) 37.97 ± 5.66 11.9 ± 2.01 ( x 106 ells) 9.10 ± 1.0 ( x 106 cells) 47.1 ± 6.06** 4.18 ± 0.36** ( x 106 ells) Time post -wound Unwound control Saline SA 1e7 CFU Blood EGFP hiGr-1 hi cell (# per femur) Supplemental Figures Figure S1. Flow cytometric analysis of EGFP cells isolated from bone marrow. (A) Gating of EGFP+ cells. Whole bone marrow cells were isolated from EGFP-lys-mice and EGFP+ cells are gated on Gr-1+ and CD11b+ cells. The vast majority of EGFPhigh cells (e.g. ~95%) and ~55% of EGFPlow cells are Gr-1+ and CD11b+, confirming they were mature PMNs. A C-kit- EGFPhigh Gr-1+ CD11b+ cells on methylcellulose medium B Day 0 Day 7 C Exponential curve fitting for estimation of rate constant of decay, k EGFP-PMN=No*e-k*(t-t) t=t Figure S2. Adoptive transfer of EGFP-PMNs for quantification of PMN half-life in tissue. (A) Lack of any proliferative capacity of FACS-sorted cells (c-kit- EGFPhigh Gr-1high CD11bhigh cells). Top figure is a representative EGFP-PMN at day 0 after plating in methylcellulose medium. By day 7 the EGFP signal has completely decayed and there is no colony formation. Bar=10μm (B) Blood EGFP-PMN numbers from wounded and S. aureus (SA)-infected (or saline-treated) C57BL/6 mice following adoptive transfer of c-kit- EGFPhigh Gr-1+ CD11b+ cells. EGFP-PMN numbers were measured by collecting blood samples from tail-vein (n=3-4 mice per group). (C) Exponential curve fitting to estimate the rate constant of EGFP-PMN decay (k) within wounded skin that best fits experimental data. Mean value of k , 0.022±0.0006 (hr-1) for Saline and 0.0059±0.0005 (hr-1) for SA 1e7 CFU. B Bone marrow c-kit A Lineage (Gr-1, TER-119, B220, CD3) C Blood Figure S3. FACS analysis of Lin- c-kit+ cell population in the bone marrow and blood in response to S. aureus infection (1x107 CFU). (A) Representative FACS plot for Lin- c-kit+ cell gating. Number of Lin- c-kit+ cells in the bone marrow (B) and blood (C) on day 1 and day 3 post-wounding and infection. Data are derived from three to six mice in each group and expressed as mean ± SEM. *, p<0.05 vs Saline control group. A B Figure S4. Expansion of bone marrow c-kit+ cells in blood and tissue culture. (A) Blood EGFP-PMN numbers following adoptive transfer of c-kit+ EGFP- cells isolated from bone marrow of EGFP-lys-mice into wounded and S. aureus (SA)-infected (or saline-treated) C57BL/6 mice. The appearance of EGFPPMNs in blood following adoptive transfer of c-kit+/EGFP- cells were measured by collecting samples from tail-vein at selected time points. In a separate group of mice, in order to deplete EGFP-PMNs appearing in the blood following transfer of c-kit+ EGFP- cells, Gr-1 mAb was injected at days 1, 3, and 5 post-S. aureus infection/wounding and blood EGFP-PMN number was measured at selected time points. (B) Effect of Gr-1 mAb on the proliferative capacity of c-kit+ cells. The c-kit+ cells were isolated from bone marrow of mice treated with Gr-1 mAb and plated in methylcellulose medium for 7 days in a colony forming assay. The c-kit+ cells from mice treated with Gr-1 mAb was not significantly altered as compared with mice treated with isotype control. A B Figure S5. Efficacy of ACK2 antibody injection (i.p) on depleting c-kit+ cells in the bone marrow. Ckit+ cell depleting anti-mouse antibody, ACK2, was injected into EGFP-lys-mice (1mg, i.p) and total number of c-kit+ cells (A) and mature PMN (B) were counted on day 5 post-antibody injection. The result was compared with mice receiving isotype control. Data represent 2 (B) to 3 (A) mice in each group. Error bars represent SEM; * , p<0.05. A Compartment model of tissue PMN under Pre Gr-1 treatment: Ppre Gr-1 Pc-kit (production by c-kit proliferation) k Ppre Gr-1 (tissue clearance) B Ppre Gr-1=mg/(g-k) (e-kt-e-gt) Figure S6. Compartment model for estimating wound PMN dynamics. (A) Pre-Gr-1 condition is modeled by balance between new PMN production by c-kit cell proliferation and wound clearance of PMN with a rate constant of k. (B) Estimation of parameters g (=0.0012 hr-1) and m (=3.58 No) based on curve fitting of estimated PpreGr-1 function (red line) into experimental data of SA-pre Gr-1 data (black circle) in Figure 6B. Figure S7. S. aureus directly induces proliferation and differentiation of c-kit+ HSPCs via MyD88dependent pathway in vitro. C-kit+ BM cells from C57Bl/6 or MyD88 knock-out mice were stimulated with heat-killed S. aureus in vitro and then plated in methylcellulose media. Fold change above baseline CFU production is presented. Data are derived from three C57Bl/6 mice and four MyD88 knock-out mice and expressed as mean ± SEM. *, p<0.01 vs MyD88 knock-out.