* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mouse genetics provides insight into folliculogenesis, fertilization
Epigenetics of neurodegenerative diseases wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Gene expression profiling wikipedia , lookup
Primary transcript wikipedia , lookup
Nutriepigenomics wikipedia , lookup
History of genetic engineering wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Genomic imprinting wikipedia , lookup
Designer baby wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
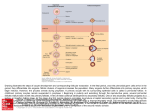
Human Reproduction Update, Vol.8, No.5 pp. 395±403, 2002 Mouse genetics provides insight into folliculogenesis, fertilization and early embryonic development Asma Amleh1 and Jurrien Dean Laboratory of Cellular and Developmental Biology, NIDDK, National Institutes of Health, Bethesda, MD 20892, USA 1 To whom correspondence should be addressed at: Laboratory of Cellular and Developmental Biology, NIDDK, Room 3133, Building 50, National Institutes of Health, Bethesda, Maryland, 20892-8028. E-mail: [email protected] After colonization of the gonad, mouse female germ cells enter into the prophase of the ®rst meiotic division as a mid-gestational hallmark of gender. Perinatally, oocytes interact with granulosa cells to form primordial follicles which, with cyclic periodicity, enter into a 3-week growth phase that culminates in meiotic maturation and ovulation. Successful fertilization in the oviduct results in the onset of embryogenesis. Genes expressed in oocytes encode maternal factors that control many of these developmental processes. The establishment of mouse models in which speci®c genes have been disrupted offers robust insights into molecular mechanisms that control oogenesis, folliculogenesis, fertilization and early embryogenesis. Although relatively few developmental circuits have been characterized in genetic detail, the ongoing revolution in mouse genetics holds great promise. These model systems provide novel information into the molecular basis of the pathways required for oocyte-speci®c processes as well as for interactions with the temporally changing environment of female germ cells. The similarities between the mouse and human genomes provide assurance that this knowledge will rapidly translate into a better understanding of human reproduction. Key words: folliculogenesis/maternal factors/meiotic maturation/oogenesis/transgenesis TABLE OF CONTENTS Introduction Origin of female germ cells Oocyte-speci®c factors affecting folliculogenesis Oocyte maturation Fertilization Early embryogenesis Summary Acknowledgements References Introduction During folliculogenesis, oocytes grow as the surrounding granulosa cells proliferate and differentiate. The subsequent maturation of the oocyte and completion of the ®rst meiotic division occurs as the oocyte is ovulated into the oviduct in preparation for fertilization. Unlike the male gonad in which seminiferous tubules are formed in the absence of germ cells, folliculogenesis does not occur in the absence of oocytes. This suggests that there are interactions between the germ and granulosa cell compartments that are critical for successful growth of a follicle. During oogenesis, there is accumulation of maternal proteins that are of importance not only for successful Ó European Society of Human Reproduction and Embryology folliculogenesis and germ cell maturation, but also for the activation of the embryonic genome and early development. Rather than a broad survey of this substantial ®eld, this review will focus on recent examples of genes critical for oocyte success, the functions of which have been elucidated by molecular biology and mouse genetics. Origin of female germ cells Mammalian germ cell lineage is established early during fetal development. In the mouse, primordial germ cells (PGC) are ®rst detected ~7.25 days after fertilization (embryonic day E7.25) as a cluster of cells identi®ed by their high content of alkaline phosphatase (Ginsburg et al., 1990). However, the germ cell lineage arises earlier and has been traced to the proximal region of the epiblast close to the extra-embryonic ectoderm (Lawson and Hage, 1994). A subset of these cells is swept into the extraembryonic mesoderm where, under the in¯uence of non cellautonomous factors, such as bone morphogenetic protein 4 (BMP4), they differentiate into PGC (Fujiwara et al., 2001). Bmp4 homozygous null mutants fail to generate PGC, and the heterozygous mutant mice have reduced number of PGC (50% that of the wild type), suggesting that the activity of BMP4 is dose dependent. Another member of the BMP superfamily (BMP8B) 395 A.Amleh and J.Dean also appears to be required for PGC generation (Ying et al., 2000) and there is evidence that BMP4 and BMP8B signalling pathways act synergistically (Ying et al., 2001). Smad 1 and 5 (vertebrate homologues of C. elegans SMA and D. melanogaster MAD proteins) are thought to act downstream of the BMP signalling pathway and their loss results in decreased numbers of primordial germ cells (Massague and Chen, 2000; Chang and Matzuk, 2001; Tremblay et al., 2001). Oct4 expression faithfully follows the germ cell lineage. Oct4 transcripts are ®rst detected in the epiblast during gastrulation (~E6.5) and later became restricted to PCG located in the extraembryonic mesoderm (Yeom et al., 1996). The germ cells subsequently enter the embryo proper and migrate to the urogenital ridge ~E10. Migration of PCG through the hind gut along the mesentery towards the future gonad is thought to be mediated by a tyrosine kinase receptor (KIT) expressed on the surface of PGC, and its ligand (KL/Steel/SCF), which is expressed in the somatic cells along the migratory pathway (Matsui et al., 1990; Keshet et al., 1991). Mutations in either the KIT receptor or ligand result in female gonadal dysgenesis and sterility (Mintz and Russell, 1957; McCoshen and McCullion, 1975). Female PGC in the gonad enter meiosis ~E13.5, an event that is a hallmark of the ovarian development. Ovarian somatic cell differentiation, unlike that of the testis, requires the presence of oocytes (McLaren, 1991). Atm (ataxia-telangiectasia-mutant) and Dazl1 (deleted-inazoospermia-like-1) are expressed in murine germ cells and are essential for PGC survival/development. Atm encodes a nuclear protein that has been implicated in double-stranded DNA break repair and cell cycle control. In male and female ATM-de®cient mice, gametogenesis is disrupted early in the leptotene stage of meiosis and the mice are sterile (Barlow et al., 1998). Dazl1 encodes cytoplasmic protein with RNA binding motifs hypothesized to affect translation. Male and female mice lacking the Dazl1 gene lose their germ cells prenatally (McNeilly et al., 2000) and are sterile, although the mechanism of germ cell loss remains to be determined. Oocyte-speci®c factors affecting folliculogenesis The female gamete grows and undergoes meiotic maturation during folliculogenesis. This process begins in the latter stages of fetal development when primordial germ cells enter meiosis and arrest at the end of prophase (dictyate in mice). Many of the molecules de®ning genetic circuits that control progression of oogenesis remain unknown. In the fetal mouse ovary, germ cells normally form clusters due to cell division with incomplete cytokinesis (Ruby et al., 1969; Pepling and Spradling, 1998). These germ-line syncytia undergo a developmentally programmed breakdown associated with invasion of pre-granulosa cells just prior to primordial follicle formation (Pepling and Spradling, 2001). In the neonatal ovary, each surviving dictyate oocyte is enclosed in a layer of ¯attened pre-granulosa cells which, in turn, are surrounded by a basal lamina to form primordial follicles (Hirsh®eld, 1991). The primordial follicles in the newborn mouse ovary represent the entire complement of germ cells available for reproduction. After birth, cohorts of primordial follicles are periodically recruited to enter into a 3-week growth phase. Concomitant with 396 the growth of oocytes, the granulosa cells transform to acquire a cuboidal shape, forming primary follicles. The proliferation of granulosa cells results in pre-antral follicles composed of multilayers of granulosa cells surrounding the oocyte within the follicular basement membrane. After puberty, further growth of follicles is accelerated by gonadotrophins, FSH, leading to the formation of antral follicles (Kumar et al., 1997). Although oocytes within large antral follicles are competent for maturation, they remain arrested due to their interaction with the surrounding granulosa cells (Bornslaeger et al., 1986). In response to the LH surge, fully grown oocytes complete the ®rst meiotic division, extrude the ®rst polar body, and become arrested at metaphase II (MII) (Richards et al., 2002). The LH surge is responsible for ovulation of the oocyte marking the end of folliculogenesis (Lee et al., 1996). The number of follicles that ovulate compared to those that are recruited for growth in any given cycle is smaller because the majority of follicles undergo atresia (Richards, 1980; Hirsh®eld, 1989). Follicles may become atretic at any stage of development, but mostly at the pre-antral and early antral stages. Death of the oocyte within the follicle occurs either intrinsically or as a consequence of follicular cell death. It remains to be determined why the majority of oocytes undergo atresia and only a few proceed to ovulation. Folliculogenesis and oogenesis are coupled processes that are co-ordinated through gap junction communication resulting in mature oocytes competent for fertilization and embryonic development. No follicle is formed without an oocyte and oocyte development is regulated by the surrounding granulosa cells. The oocyte secretes signals that induce follicle formation, prompt granulosa cell proliferation, regulate steroidogenesis, and maintain the architecture of the developing follicle. Similarly, signals from granulosa cells regulate meiotic arrest, promote oocyte growth and facilitate the resumption of meiosis and oocyte maturation (Eppig, 2001; Epifano and Dean, 2002). Determining the roles of genes expressed in the oocyte should provide insight into critical events involved in the ovarian development. The few genes that have been identi®ed as exclusively expressed in the oocyte appear to function as multipurpose factors throughout folliculogenesis and/or early embryogenesis (Figure 1). Factor In the Germline, alpha Factor In the Germline, alpha (FIGa), a germ-cell speci®c basic helix±loop±helix (bHLH) transcription factor, has been implicated in the coordinate expression of the three zona pellucida genes (Zp1, Zp2, Zp3) (Liang et al., 1997). In addition, female, but not male, mice that are de®cient in FIGa are infertile (Soyal et al., 2000). No primordial follicles are formed in the ovaries of the newborn Figa null females despite normal embryonic gonadogenesis and oogenesis. It has been demonstrated that comparable numbers of null and normal oocytes reach the pachytene and diplotene stages of meiotic prophase. However, within the ®rst week after birth, ovaries of the Figa null females are devoid of oocytes. Whether FIGa is involved in oocyte survival or in regulating the initial granulosa±oocyte interactions remains to be determined. As expected, ZP1, ZP2 and ZP3 transcripts are not detected in ovaries of the Figa null females when examined prior to oocyte depletion. These results indicate that FIGa plays a key regulatory role in the expression of at least two oocyte-speci®c Mouse models for human reproduction Figure 1. Genes implicated in folliculogenesis. Perinatally, FIGa, a basic helix±loop±helix transcription factor, is required for the formation of the primordial follicle. Progression to the secondary stage of folliculogenesis requires germ-cell speci®c growth factors including GDF9 and BMP15. Oocytes lacking CONNEXIN37 are unable to form effective gap junctions between germ and granulosa cells and do not mature beyond the early antral stage of folliculogenesis. The genes encoding these factors are indicated as Figa (Factor in the Germline, alpha), Gdf9 (Growth and Differentiation Factor 9), Bmp15 (Bone Morphogenetic Factor 15) and Cx37 (Connexin 37). pathways, those that initiate folliculogenesis and those that express the three genes encoding the zona pellucida. The persistence of FIGa transcripts in adult ovaries suggests that it may regulate additional pathways. Furthermore, the presence of FIGa transcripts (and, presumably protein) in fetal ovaries at E13 may in¯uence genes that regulate the earliest events of oogenesis. Growth and differentiation factor 9 As oocytes enter the growth phase of oogenesis, they produce GDF9, which promotes early granulosa cell proliferation and induces differentiation of cumulus cells later at the antral stage of folliculogenesis (Elvin et al., 1999b). GDF9 is a member of the TGFb super family of secreted signalling proteins. In ovaries of female mice that are de®cient in GDF9, follicles do not grow beyond the primary stage, while oocytes grow in size, but eventually degenerate within the primary follicle (Dong et al., 1996). Interestingly, these granulosa cells are competent to undergo luteinization while the fully-grown oocyte never acquires the competence for maturation. Recombinant mouse GDF9 induces the expression of cyclooxygenase 2 (COX-2), STeroidogenic Acute Regular protein (StAR), and increases granulosa cell progesterone synthesis in the absence of FSH in vitro (Carabatsos et al., 2000; Dong et al., 1996). Furthermore, GDF9 promotes cumulus expansion in oocytectomized follicles by inducing the hyaluronidase synthetase gene and by inhibiting the expression of urokinase plasminogen activator (Elvin et al., 1999a). Thus, it appears that GDF9 plays a critical role in the successful growth and differentiation of follicles. Bone morphogenetic protein 15 Bmp15, also known as Gdf9b, is an X-linked gene that is expressed exclusively in oocytes, and its protein product has a high degree of homology (52%) with GDF9. Similar to Gdf9, Bmp15 gene expression is ®rst detected in oocytes within primary follicles and its protein product stimulates granulosa cell proliferation (Carabatsos et al., 1998; Dube et al., 1998; Otsuka et al., 2000). In addition, BMP15 selectively inhibits FSHinduced progesterone production, but not FSH-induced estradiol production, due in part to the regulation of FSH receptor transcription (Otsuka et al., 2001). Female mice that are de®cient in BMP15 are subfertile with reduced litter size due to ovulatory defects (Yan et al., 2001). Furthermore, not all BMP15 de®cient oocytes, when fertilized, are competent for preimplantation embryonic development. By examining and characterizing mice with Bmp15±/±, Gdf9+/± or Bmp15±/±, Gdf9±/± double mutants, it has become clear that BMP15 and GDF9 proteins have synergistic roles in ovarian function. Unlike Bmp15±/± null female mice, sheep that are de®cient in BMP15 are sterile due to an arrest in folliculogenesis and phenocopy female mice de®cient in GDF9. Interestingly, sheep heterozygous for a Bmp15 null allele display increased fertility compared to normal, suggesting that fertility is regulated by BMP15 in a dosage sensitive manner (Galloway et al., 2000). Species-speci®c models have been proposed to incorporate these disparate observations. In mice, GDF9 homodimers would be the most bioactive based on the phenotype of mouse Gdf9±/± and Bmp15±/± as well as the results of an in-vitro bioassay which examined the activity of GDF9 and BMP15 homodimers. In sheep, the data suggest that BMP15 homodimers are the most bioactive compared to either GDF9 homodimers or BMP15/ GDF9 heterodimers (Yan et al., 2001). Oocyte maturation Meiotic maturation The acquisition of meiotic maturation competence occurs in two steps during oocyte growth. The germ cell ®rst gains the ability to undergo germinal vesicle breakdown (GVBD) and progress to 397 A.Amleh and J.Dean metaphase I (MI) (Canipari et al., 1984; Chesnel and Eppig, 1995), and then the ability to progress to MII (Sorensen and Wassarman, 1976; Wickramasinghe et al., 1991). Resumption and completion of meiosis in the oocyte involve orchestration of three major factors: MPF (Maturation Promoting Factor), MOS (a proto-oncogene), and MAPK (Mitogen Activated Protein Kinase). MPF is a protein complex composed of a catalytic subunit, p34cdc2, and a regulatory subunit, CYCLIN B (O'Keefe et al., 1991). In mouse, MPF activity precedes GVBD and is believed to initiate a cascade of events leading to protein phosphorylation, which drives the oocyte through meiotic progression (Araki et al., 1996; Choi et al., 1996). However, a transient decrease in MPF, associated with attenuated CYCLIN B, is necessary for the extrusion of the ®rst polar body. Thus, the rates of CYCLIN B synthesis and degradation determine the timing of the major events during mouse oocyte meiotic maturation (Ledan et al., 2001). It appears that MPF requires the phosphatase action of Cdc25b to become functionally active. Ovaries of female mice lacking Cdc25b protein provide oocytes that fail to undergo GVBD despite their passage through normal folliculogenesis (Lincoln et al., 2002). The proto-oncogene MOS, which is a member of the serine/ threonine protein kinase family, appears to be required for the activation of MAPK. MOS mRNA in the growing oocyte is transcriptionally dormant with short polyA tails (Paynton and Bachvarova, 1994; Salles et al., 1992). Upon GVBD, the polyA tail is elongated by polyadenylation and MOS mRNA becomes translationally activated. Subsequently, MOS proteins appear to be required for the activation of MAPK (Colledge et al., 1994; Hashimoto et al., 1994). The MOS/MAPK pathway is responsible for proper spindle formation at MI and MII, repression of DNA replication at the MI-MII transition and maintenance of the MII arrest (Verlhac et al., 1993; Colledge et al., 1994; Gebauer et al., 1994; Hashimoto et al., 1994; Araki et al., 1996). Gap junctions During folliculogenesis, oocytes and the surrounding granulosa cells extend cellular processes towards one another and establish gap junctions by which the cells in the follicle communicate. While these gap junctions maintain the communication between the oocyte and granulosa cells implicated in meiotic arrest, there is evidence that they also mediate activating events leading to oocyte maturation (Eppig, 1991). Gap junctions are formed by connexin proteins, some of which are ubiquitous and others which have more restricted spatial and developmental patterns of expression (White and Paul, 1999). CONNEXIN 37 (CX37) is expressed by the oocyte at all stages of folliculogenesis and participates in the formation of these gap junctions. In CX37de®cient female mice, follicle development fails at the pre-antral± antral transition resulting in sterile females (Simon et al., 1997). It has been shown that oocytes released from pre-antral follicles of Cx37 null mice respond to a protein phosphatase inhibitor (okadiac acid) and enter M phase of meiosis (Carabatsos et al., 2000). However, Cx37 null oocytes are unable to maintain the M phase after removal of okadiac acid, suggesting that acquisition of oocyte cytoplasmic maturation is dependent on gap junction formation, but that nuclear maturation is not. Mice with targeted disruption of Cx43 (expressed in granulosa cells) are de®cient in germ cells. Those that are present form follicles, but do not 398 progress beyond the primary or early secondary stage. In the absence of CX43, intercellular coupling between granulosa cells is reduced, leading to an early arrest in folliculogenesis and severe disruption of germ cells (Ackert et al., 2001). Zona pellucida An important component of growing follicles is the extracellular zona pellucida that separates the oocyte from the surrounding granulosa cells. The zona pellucida is synthesized in oocytes and is ®rst detected in primary follicles as patches of coalesced extracellular matrix. As oocytes grow, the zona pellucida increases in width from 3 mm in the secondary follicle to 7 mm in the early antral follicle (Dietl, 1989). The matrix is composed of three sulphated glycoproteins, ZP1, ZP2, ZP3. ZP2 and ZP3 are major, approximately equal components, and ZP1 represents 10± 15% of the zona mass (Bleil and Wassarman, 1980b). The role of the individual zona proteins in the formation of the matrix has been investigated using mouse lines with null mutations in each of the single copy Zp1, Zp2 or Zp3 genes (Liu et al., 1996; Rankin et al., 1999, 1996, 2001). Although normally the zona matrix is composed of all three proteins (ZP1/ZP2/ZP3), a zona matrix can be formed with either ZP1/ZP3 or ZP2/ZP3, suggesting that only ZP3 is essential for zona pellucida formation. However, the ZP1/ ZP3 zona matrix is particularly thin and does not persist past the formation of the antral stage of folliculogenesis. Oocytes lacking ZP2 or ZP3 develop, but the latter stages of folliculogenesis are perturbed and relatively few zona-free oocytes are recovered in the oviduct after gonadotrophin-stimulated ovulation. While these zona-free oocytes can be fertilized in vitro and progress to the blastocyst stage, no live births have been observed after transfer to pseudopregnant females. These results suggest that the morphologic abnormalities observed during folliculogenesis in the Zp2 and Zp3 null mutants adversely affect the developmental competence of the zona-free oocytes after fertilization. Mice lacking ZP1 have a fairly robust-looking zona matrix, but there is ectopic localization of granulosa cells in the perivitelline space between the oocyte's plasma membrane and the inner aspect of the zona pellucida in ~10% of the growing follicles. ZP1 is the only zona protein that forms inter-molecular disulphide bonds, and may therefore provide structural integrity to the zona pellucida beyond its relatively minor contribution to the mass of the zona pellucida. Fertilization After ovulation, the zona pellucida mediates both the initial sperm±oocyte recognition and triggers the acrosome reaction. There is in-vitro evidence that the sperm recognizes ZP3 within the zona matrix (Bleil and Wassarman, 1980a) and data have been advanced suggesting a role for O-linked oligosaccharide side chains (Bleil and Wassarman, 1980a; Florman and Wassarman, 1985). Different laboratories have implicated terminal a1,3galactose (Bleil and Wassarman, 1988) or N-acetylglucosamine (Miller et al., 1992) in mediating this interaction. However, more recent genetic studies have not con®rmed the primacy of ZP3 as a sperm receptor (Rankin et al., 1998) and mice lacking either the galactosyltransferase required for the addition of a1,3-galactose (Thall et al., 1995), or the galactosyltransferase isoform thought Mouse models for human reproduction Figure 2. Maternal proteins affecting early development. During meiotic maturation, the oocyte genome becomes transcriptionally silent, fertilization occurs with a transcriptionally inert sperm and activation of the embryonic genome does not occur until the transition between the 1 and 2-cell stages. Thus, events during the latter stages of meiotic maturation and early embryogenesis must be controlled by pre-existing maternal factors which can be activated by a variety of mechanisms. MPF (Maturation Promoting Factor) is required for meiotic maturation; MOS, a proto-oncogene, and MAPK (Mitogen Activated Protein Kinase) are required for meiotic spindle formation; SPIN (SPINDLIN), present in growing oocytes, persists until the 4 cell stage of embryogenesis and is associated with the ®rst mitotic spindle; HSF1 (Heat-shock Factor 1) is required for early embryogenesis; and embryos lacking MATER (Maternal Antigen That Embryos Require) do not progress beyond the 2-cell stage. to bind the terminal N-acetylglucosamine residue (Asano et al., 1997; Lu and Shur, 1997), remain fertile. Normally, human sperm will not bind to mouse oocytes (Bedford, 1977). Therefore, to genetically determine if ZP3 was responsible for the taxon-order speci®city of sperm binding, transgenic mice expressing human ZP3 were established and bred into a Zp3 null line (Rankin et al., 1998). The expression of human ZP3 protein in mouse oocytes rescues the mouse Zp3 null phenotype by restoring the integrity of the zona matrix. However, there is no change in the speci®city of sperm binding: mouse sperm bind and fertilize the `humanized' mouse oocyte and human sperm do not bind despite the presence of human ZP3. Given the importance ascribed to carbohydrate side chains in sperm binding, it seemed possible that human ZP3 expressed in mouse oocytes is functionally converted from human to mouse speci®city by post-translational modi®cation. However, based on mobility in sodium dodecyl sulphate±polyacrylamide gel electrophoresis, human ZP3 expressed in mouse oocytes is posttranslationally modi®ed to the same extent as native human ZP3 (64 kDa) and distinctly different from native mouse ZP3 (83 kDa). While unable to discount subtle post-translational modi®cations, it now seems more likely that zona components beyond ZP3 are required to render human sperm binding. Mouse lines expressing additional human zona proteins are in the process of being evaluated. Once the oocyte has been fertilized, cortical granules located in the periphery exocytose into the perivitelline space. This process modi®es the zona pellucida so that no more sperm bind and sperm within the perivitelline space are unable to fuse with the oocyte's plasma membrane. The zona modi®cation involves cleavage of ZP2 into several N-terminal polypeptides (21±31 kDa) that remain bound to the parental ZP2 by disulphide bonds (Bleil et al., 1981; Greenhouse et al., 1999). Whether the cleavage of ZP2 is causative or re¯ective of the block to polyspermy remains to be resolved. Moreover there may be other, yet to be described, modi®cations of the zona matrix that are important for the block to polyspermy. Early embryogenesis At fertilization, both male and female gametes are transcriptionally inert and the earliest stages of embryogenesis are dependent on maternal components stored within the oocyte (Bachvarova and De Leon, 1980; Rosenthal and Wilt, 1986; Paynton et al., 1988; Stutz et al., 1998). While some maternal proteins are immediately available for early development, others need to be translated from dormant maternal mRNA after activation by cytoplasmic polyadenylation (Huarte et al., 1987). Initially these untranslated maternal mRNA are bound by cytoplasmic proteins, making them inaccessible to the translation apparatus (Curtis et al., 1995). Subsequent, post-translational modi®cations of RNA binding proteins release mRNA and make them available for polyadenylation and translation (Richter et al., 1990; Mendez et al., 2000). Additional factors that bind to the 3¢ untranslated region (UTR), including the nuclear polyadenylation signal (AAUAAA) and the cytoplasmic polyadenylation element (UUUUUAU), are then required for the correct temporal translational control of these transcripts. These newly synthesized maternal factors along with pre-existing proteins, some of which have undergone post-translational modi®cations (phosphorylation, glycosylation, proteolytic processing), are responsible for the early developmental events (Cascio and Wassarman, 1982; Howlett and Bolton, 1985; Latham et al., 1991; Oh et al., 1997) As embryonic development proceeds, maternal components decay and the process of embryogenesis becomes increasingly dependent on the expression of the embryonic genome. De-novo embryonic gene transcription is ®rst detected in the male pronucleus in the late 1-cell embryo (Bouniol et al., 1995), but the major activation of the embryonic genome does not occur until the 2-cell stage (Flach et al., 1982; Schultz, 1993). Many of 399 A.Amleh and J.Dean the early events of development, including activation of embryonic transcription, are mediated by maternal proteins, but only a small number of maternal-effect genes have been identi®ed in mammals (Figure 2). SPINDLIN Some proteins synthesized from maternal mRNA have relatively long half-lives and can remain at detectable levels until the morula stage of development (Pratt et al., 1983; Richoux et al., 1991). However, other proteins are only expressed for a short period in a stage-speci®c manner. For example, the ®rst cell cleavage requires the phosphorylation of SPINDLIN (SPIN), which is associated with the ®rst mitotic spindle formation (Oh et al., 1997). The SPIN transcripts and their products are detected in unfertilized oocytes and 2-cell embryos, but not in 8-cell embryos. There are three different SPIN transcripts which have the same open reading frame, but differ in their 3¢ UTR and the length of their polyA tails. The shortest message (0.8 Kb) is expressed in oocytes prior to fertilization whereas the other two transcripts are more ubiquitously present. Unlike the two longer transcripts, the 0.8 kb transcript has no cytoplasmic polyadenylation element (CPE) motif which suggests that the 3¢ UTR may account for differences in translation (Oh et al., 2000). Maternal Antigen That Embryos Require (MATER) Recently, the gene encoding MATER was characterized and mouse lines lacking the protein were established (Tong and Nelson, 1999; Tong et al., 2000). While homozygous null Mater males have normal fertility, females are sterile. These mice have normal folliculogenesis and their ovulated eggs can be fertilized. However, embryos derived from MATER-de®cient females do not grow beyond the 2-cell stage and exhibit a decrease in transcriptional activity. Normally, there are two distinct phases of transcriptional activities, early and late, that can be discriminated by the sensitivity to a-amanitin treatment, known to inhibit the formation of mRNA precursors (Worrad et al., 1994; Worrad and Schultz, 1997). A stage-speci®c protein, the transcriptionrequiring complex (TRC), is synthesized during the early phase of zygotic gene expression (Conover et al., 1991). The TRC complex has been detected in embryos generated by females lacking MATER, albeit at only 60% of the normal amounts. These results suggest that zygotic genome activation is initiated (at least partially) in embryos lacking MATER during early embryonic activation. Although the primary structure of MATER has been deduced from full-length cDNA, little is known about its function in early embryogenesis. Heat-Shock Factor-1 (HSF1) Heat-Shock Factor-1 (HSF1) is known to activate stress-inducible genes such as Hsp70.1 which is among the ®rst zygotic genes to be expressed after fertilization (Bensaude et al., 1983; Christians et al., 1997). Mice lacking HSF1 proteins are viable, but females are infertile due to failed preimplantation development (Christians et al., 2000). Similar to oocytes de®cient in MATER, germ cells from Hsf1 null females have normal folliculogenesis and can be 400 fertilized. However, the majority of embryos generated by HSF1de®cient females do not develop beyond the 1-cell stage. When females lacking HSF1 are crossed with males that carry a Hsp70 promoter-luciferase transgene, E1.5 embryos exhibited luciferase activity suggesting that at least some zygotic transcription activity can be initiated in the absence of HSF1. Whether HSF1 is required for complete activation of the zygotic genome or embryonic survival remains to be determined. Summary Female germ cells are programmed for success. Using developmentally controlled, oocyte-speci®c genes, they ensure correct prenatal sexual identity, growth and survival during folliculogenesis, successful fertilization and activation of the embryonic genome. Mouse genetics have provided unique insights into the function of multiple genes involved in these processes and will serve as a springboard for future research. In particular, it will be useful to further characterize the role of BMP4 and OCT4 in the establishment of the female germ cell lineage and the mechanisms by which DAZL1 preserves germ cells perinatally. While signi®cant advances have been made in identifying oocytespeci®c factors that affect folliculogenesis (e.g. FIGa, GDF9, BMP15, CX37) and meiotic maturation (e.g. MPF, MOS MAPK), clearly additional genes are involved. The near completion of the human and mouse genome projects offers the promise of accelerated gene discovery. In addition to identifying the role of individual genes, the use of high throughput genomic screens holds forth the prospect of de®ning novel genetic pathways (e.g. downstream targets of FIGa). Particularly exciting avenues of research include evolving investigations into the role of maternal effect genes (e.g. Spindlin, Mater, Hsf1) in early embryogenesis. Induced null mutations and allelic series of mouse genes that result in phenotypes affecting germ cell development and folliculogenesis will continue to advance the boundaries of our knowledge. Current technologies that emphasize genetic approaches in model systems should rapidly translate into a better understanding of human biology. Acknowledgements We appreciate the critical reading of the manuscript by Drs Teruko Taketo, Olga Epifano and Holly Davies and apologize to our colleagues whose work was not cited because of space limitations. Portions of this review have been covered in previous publications of the authors. References Ackert, C.L., Gittens, J.E., O'Brien, M.J., Eppig, J.J. and Kidder, G.M. (2001) Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol., 233, 258±270. Araki, K., Naito, K., Haraguchi, S., Suzuki, R., Yokoyama, M., Inoue, M., Aizawa, S., Toyoda, Y. and Sato, E. (1996) Meiotic abnormalities of cmos knockout mouse oocytes: activation after ®rst meiosis or entrance into third meiotic metaphase. Biol. Reprod., 55, 1315±1324. Asano, M., Furukawa, K., Kido, M., Matsumoto, S., Umesaki, Y., Kochibe, N. and Iwakura, Y. (1997) Growth retardation and early death of beta-1,4galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J., 16, 1850±1857. Mouse models for human reproduction Bachvarova, R. and De Leon, V. (1980) Polyadenylated RNA of mouse ova and loss of maternal RNA in early development. Dev. Biol., 74, 1±8. Barlow, C., Liyanage, M., Moens, P.B., Tarsounas, M., Nagashima, K., Brown, K., Rottinghaus, S., Jackson, S.P., Tagle, D., Ried, T. et al. (1998) Atm de®ciency results in severe meiotic disruption as early as leptonema of prophase I. Development, 125, 4007±4017. Bedford, J.M. (1977) Sperm/egg interaction: the speci®city of human spermatozoa. Anat. Rec., 188, 477±488. Bensaude, O., Babinet, C., Morange, M. and Jacob, F. (1983) Heat shock proteins, ®rst major products of zygotic gene activity in mouse embryo. Nature, 305, 331±333. Bleil, J.D. and Wassarman, P.M. (1980a) Mammalian sperm±egg interaction: identi®cation of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell, 20, 873±882. Bleil, J.D. and Wassarman, P.M. (1980b) Structure and function of the zona pellucida: identi®cation and characterization of the proteins of the mouse oocyte's zona pellucida. Dev. Biol., 76, 185±202. Bleil, J.D. and Wassarman, P.M. (1988) Galactose at the nonreducing terminus of O-linked oligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein's sperm receptor activity. Proc. Natl Acad. Sci. USA, 85, 6778±6782. Bleil, J.D., Beall, C.F. and Wassarman, P.M. (1981) Mammalian sperm±egg interaction: fertilization of mouse eggs triggers modi®cation of the major zona pellucida glycoprotein, ZP2. Dev. Biol., 86, 189±197. Bornslaeger, E.A., Mattei, P. and Schultz, R.M. (1986) Involvement of cAMPdependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev. Biol., 114, 453±462. Bouniol, C., Nguyen, E. and Debey, P. (1995) Endogenous transcription occurs at the 1-cell stage in the mouse embryo. Exp. Cell Res., 218, 57± 62. Canipari, R., Palombi, R., Riminucci, M. and Mangia, F. (1984) Early programming of maturation competence in mouse oogenesis. Dev. Biol., 102, 519±524. Carabatsos, M.J., Elvin, J., Matzuk, M.M. and Albertini, D.F. (1998) Characterization of oocyte and follicle development in growth differentiation factor-9-de®cient mice. Dev. Biol., 204, 373±384. Carabatsos, M.J., Sellitto, C., Goodenough, D.A. and Albertini, D.F. (2000) Oocyte±granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev. Biol., 226, 167±179. Cascio, S.M. and Wassarman, P.M. (1982) Program of early development in the mammal: post-transcriptional control of a class of proteins synthesized by mouse oocytes and early embryos. Dev. Biol., 89, 397±408. Chang, H. and Matzuk, M.M. (2001) Smad5 is required for mouse primordial germ cell development. Mech. Dev., 104, 61±67. Chesnel, F. and Eppig, J.J. (1995) Induction of precocious germinal vesicle breakdown (GVB) by GVB-incompetent mouse oocytes: possible role of mitogen-activated protein kinases rather than p34cdc2 kinase. Biol. Reprod., 52, 895±902. Choi, T., Rulong, S., Resau, J., Fukasawa, K., Matten, W., Kuriyama, R., Mansour, S., Ahn, N. and Vande Woude, G.F. (1996) Mos/mitogenactivated protein kinase can induce early meiotic phenotypes in the absence of maturation-promoting factor: a novel system for analyzing spindle formation during meiosis I. Proc. Natl Acad. Sci. USA, 93, 4730± 4735. Christians, E., Michel, E., Adenot, P., Mezger, V., Rallu, M., Morange, M. and Renard, J.P. (1997) Evidence for the involvement of mouse heat shock factor 1 in the atypical expression of the HSP70.1 heat shock gene during mouse zygotic genome activation. Mol. Cell Biol., 17, 778±788. Christians, E., Davis, A.A., Thomas, S.D. and Benjamin, I.J. (2000) Maternal effect of hsf1 on reproductive success. Nature, 407, 693±694. Colledge, W.H., Carlton, M.B., Udy, G.B. and Evans, M.J. (1994) Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature, 370, 65±68. Conover, J.C., Temeles, G.L., Zimmermann, J.W., Burke, B. and Schultz, R.M. (1991) Stage-speci®c expression of a family of proteins that are major products of zygotic gene activation in the mouse embryo. Dev. Biol., 144, 392±404. Curtis, D., Lehmann, R. and Zamore, P.D. (1995) Translational regulation in development. Cell, 81, 171±178. Dietl, J. (1989) Ultrastructural aspects of the developing mammalian zona pellucida. In Dietl, J. (ed.), The Mammalian Egg Coat. Springer-Verlag, Berlin, pp. 49±60. Dong, J., Albertini, D.F., Nishimori, K., Kumar, T.R., Lu, N. and Matzuk, M.M. (1996) Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature, 383, 531±535. Dube, J.L., Wang, P., Elvin, J., Lyons, K.M., Celeste, A.J. and Matzuk, M.M. (1998) The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol. Endocrinol., 12, 1809±1817. Elvin, J., Clark, A.T., Wang, P., Wolfman, N.M. and Matzuk, M.M. (1999a) Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol. Endocrinol., 13, 1035±1048. Elvin, J., Yan, C., Wang, P., Nishimori, K. and Matzuk, M.M. (1999b) Molecular characterization of the follicle defects in the growth differentiation factor 9-de®cient ovary. Mol. Endocrinol., 13, 1018±1034. Epifano, O. and Dean, J. (2002) Genetic control of early folliculogenesis in mice. Trends Endocrinol. Metab., 13, 169±173. Eppig, J.J. (1991) Intercommunication between mammalian oocytes and companion somatic cells. Bioessays, 13, 569±574. Eppig, J.J. (2001) Oocyte control of ovarian follicular development and function in mammals. Reproduction, 122, 829±938. Flach, G., Johnson, M.H., Braude, P., Taylor, R.A.S. and Bolton, V.N. (1982) The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J., 1, 681±686. Florman, H.M. and Wassarman, P.M. (1985) O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell, 41, 313±324. Fujiwara, T., Dunn, N.R. and Hogan, B.L. (2001) Bone morphogenetic protein 4 in the extraembryonic mesoderm is required for allantois development and the localization and survival of primordial germ cells in the mouse. Proc. Natl Acad. Sci. USA, 98, 13739±13744. Galloway, S.M., McNatty, K.P., Cambridge, L.M., Laitinen, M.P., Juengel, J.L., Jokiranta, T.S., McLaren, R.J., Luiro, K., Dodds, K.G., Montgomery, G.W. et al. (2000) Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosagesensitive manner. Nat. Genet., 25, 279±283. Gebauer, F., Xu, W., Cooper, G.M. and Richter, J.D. (1994) Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J., 13, 5712. Ginsburg, M., Snow, M.H.L. and McLaren, A. (1990) Primordial germ cells in the mouse embryo during gastrulation. Development, 110, 521±528. Greenhouse, S., Castle, P.E. and Dean, J. (1999) Antibodies to human ZP3 induce reversible contraception in transgenic mice with `humanized' zonae pellucidae. Hum. Reprod., 14, 593±600. Hashimoto, N., Watanabe, N., Furuta, Y., Tamemoto, H., Sagata, N., Yokoyama, M., Okazaki, K., Nagayoshi, M., Takeda, N. and Ikawa, Y. (1994) Parthenogenetic activation of oocytes in c-mos-de®cient mice. Nature, 370, 68±71. Hirsh®eld, A.N. (1989) Rescue of atretic follicles in vitro and in vivo. Biol. Reprod., 40, 181±190. Hirsh®eld, A.N. (1991) Development of follicles in the mammalian ovary. Int. Rev. Cytol., 124, 43±101. Howlett, S.K. and Bolton, V.N. (1985) Sequence and regulation of morphological and molecular events during the ®rst cell cycle of mouse embryogenesis. J. Embryol. Exp. Morphol., 87, 175±206. Huarte, J., Belin, D., Vassalli, A., Strickland, S. and Vassalli, J.D. (1987) Meiotic maturation of mouse oocytes triggers the translation and polyadenylation of dormant tissue-type plasminogen activator mRNA. Genes Dev., 1, 1201±1211. Keshet, E., Lyman, S.D., Williams, D.E., Anderson, D.M., Jenkins, N.A., Copeland, N.G. and Parada, L.F. (1991) Embryonic RNA expression patterns of the c-kit receptor and its cognate ligand suggest multiple functional roles in mouse development. EMBO J., 10, 2425±2435. Kumar, T.R., Wang, Y., Lu, N. and Matzuk, M.M. (1997) Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet., 15, 201±204. Latham, K.E., Garrels, J.I., Chang, C. and Solter, D. (1991) Quantitative analysis of protein synthesis in mouse embryos. I. Extensive reprogramming at the one- and two-cell stages. Development, 112, 921± 932. Lawson, K.A. and Hage, W.J. (1994) Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found. Symp., 182, 68±84. Ledan, E., Polanski, Z., Terret, M.E. and Maro, B. (2001) Meiotic maturation of the mouse oocyte requires an equilibrium between cyclin B synthesis and degradation. Dev. Biol., 232, 400±413. Lee, S.L., Sadovsky, Y., Swirnoff, A.H., Polish, J.A., Goda, P., Gavrilina, G. and Milbrandt, J. (1996) Luteinizing hormone de®ciency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science, 273, 1219±1221. Liang, L.-F., Soyal, S.M. and Dean, J. (1997) FIGa, a germ cell speci®c 401 A.Amleh and J.Dean transcription factor involved in the coordinate expression of the zona pellucida genes. Development, 124, 4939±4949. Lincoln, A.J., Wickramasinghe, D., Stein, P., Schultz, R.M., Palko, M.E., De Miguel, M.P., Tessarollo, L. and Donovan, P.J. (2002) Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat. Genet., 30, 446±449. Liu, C., Litscher, E.S., Mortillo, S., Sakai, Y., Kinloch, R.A., Stewart, C.L. and Wassarman, P.M. (1996) Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc. Natl Acad. Sci. USA, 93, 5431±5436. Lu, Q. and Shur, B.D. (1997) Sperm from b1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development, 124, 4121±4131. Massague, J. and Chen, Y.G. (2000) Controlling TGF-beta signaling. Genes Dev., 14, 627±644. Matsui, Y., Zsebo, K.M. and Hogan, B.L. (1990) Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for ckit. Nature, 347, 667±669. McCoshen, J.A. and McCullion, D.J. (1975) A study of the primordial germ cells during their migratory phase in Steel mutant mice. Experientia, 31, 589±590. McLaren, A. (1991) Development of the mammalian gonad: the fate of the supporting cell lineage. Bioessays, 13, 151±156. McNeilly, J.R., Saunders, P.T., Taggart, M., Cran®eld, M., Cooke, H.J. and McNeilly, A.S. (2000) Loss of oocytes in Dazl knockout mice results in maintained ovarian steroidogenic function but altered gonadotropin secretion in adult animals. Endocrinology, 141, 4284±4294. Mendez, R., Murthy, K.G., Ryan, K., Manley, J.L. and Richter, J.D. (2000) Phosphorylation of CPEB by Eg2 mediates the recruitment of CPSF into an active cytoplasmic polyadenylation complex. Mol. Cell, 6, 1253±1259. Miller, D.J., Macek, M.B. and Shur, B.D. (1992) Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm±egg binding. Nature, 357, 589±593. Mintz, B. and Russell, E.S. (1957) Gene induced embryological modi®cations of primordial germ cells in the mouse. J. Exp. Zool., 134, 207±237. O'Keefe, S.J., Kiessling, A.A. and Cooper, G.M. (1991) The c-mos gene product is required for cyclin B accumulation during meiosis of mouse eggs. Proc. Natl Acad. Sci. USA, 88, 7869±7872. Oh, B., Hwang, S., McLaughlin, J., Solter, D. and Knowles, B.B. (2000) Timely translation during the mouse oocyte-to-embryo transition. Development, 127, 3795±3803. Oh, B., Hwang, S.Y., Solter, D. and Knowles, B.B. (1997) Spindlin, a major maternal transcript expressed in the mouse during the transition from oocyte to embryo. Development, 124, 493±503. Otsuka, F., Yamamoto, S., Erickson, G.F. and Shimasaki, S. (2001) Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J. Biol. Chem., 276, 11387±11392. Otsuka, F., Yao, Z., Lee, T., Yamamoto, S., Erickson, G.F. and Shimasaki, S. (2000) Bone morphogenetic protein-15: identi®cation of target cells and biological functions. J. Biol. Chem., 275, 39523±39528. Paynton, B.V. and Bachvarova, R. (1994) Polyadenylation and deadenylation of maternal mRNAs during oocyte growth and maturation in the mouse. Mol. Reprod. Dev., 37, 172±180. Paynton, B.V., Rempel, R. and Bachvarova, R. (1988) Changes in state of adenylation and time course of degradation of maternal mRNAs during oocyte maturation and early embryonic development in the mouse. Dev. Biol., 129, 304±314. Pepling, M.E. and Spradling, A.C. (1998) Female mouse germ cells form synchronously dividing cysts. Development, 125, 3323±3328. Pepling, M.E. and Spradling, A.C. (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol., 234, 339±351. Pratt, H.P.M., Bolton, V.N. and Gridgeon, K.A. (1983) The legacy from the oocyte and its role in controlling early development in the mouse embryo. Ciba Found. Symp., 98, 197±227. Rankin, T., Familari, M., Lee, E., Ginsberg, A.M., Dwyer, N., BlanchetteMackie, J., Drago, J., Westphal, H. and Dean, J. (1996) Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development, 122, 2903±2910. Rankin, T., Talbot, P., Lee, E. and Dean, J. (1999) Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development, 126, 3847±3855. Rankin, T.L., Tong, Z.-B., Castle, P.E., Lee, E., Gore-Langton, R., Nelson, L.M. and Dean, J. (1998) Human ZP3 restores fertility in Zp3 null mice 402 without affecting order-speci®c sperm binding. Development, 125, 2415± 2424. Rankin, T.L., O'Brien, M., Lee, E., Wigglesworth, K.E.J.J. and Dean, J. (2001) Defective zonae pellucidae in Zp2 null mice disrupt folliculogenesis, fertility and development. Development, 128, 1119± 1126. Richards, J.S. (1980) Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol. Rev., 60, 51±89. Richards, J.S., Russell, D.L., Ochsner, S. and Espey, L.L. (2002) Ovulation: new dimensions and new regulators of the in¯ammatory-like response. Annu. Rev. Physiol., 64, 69±92. Richoux, V., Renard, J.P. and Babinet, C. (1991) Synthesis and developmental regulation of an egg speci®c mouse protein translated from maternal mRNA. Mol. Reprod. Dev., 28, 218±229. Richter, J.D., Paris, J. and McGrew, L.L. (1990) Maternal mRNA expression in early development: regulation at the 3¢ end. Enzyme, 44, 129±146. Rosenthal, E.T. and Wilt, F.H. (1986) Patterns of maternal messenger RNA accumulation and adenylation during oogenesis in Urechis caupo. Dev. Biol., 117, 55±63. Ruby, J.R., Dyer, R.F. and Skalke, R.C. (1969) The occurrence of intercellular bridges during oogenesis in the mouse. J. Morphol., 127, 307±340. Salles, F.J., Darrow, A.L., O'Connell, M.L. and Strickland, S. (1992) Isolation of novel murine maternal mRNAs regulated by cytoplasmic polyadenylation. Genes Dev., 6, 1202±1212. Schultz, R.M. (1993) Regulation of zygotic gene activation in the mouse. Bioessays, 15, 531±538. Simon, A.M., Goodenough, D.A., Li, E. and Paul, D.L. (1997) Female infertility in mice lacking connexin 37. Nature, 385, 525±529. Sorensen, R.A. and Wassarman, P.M. (1976) Relationship between growth and meiotic maturation of the mouse oocyte. Dev. Biol., 50, 531±536. Soyal, S.M., Amleh, A. and Dean, J. (2000) FIGa, a germ-cell speci®c transcription factor required for ovarian follicle formation. Development, 127, 4645±4654. Stutz, A., Conne, B., Huarte, J., Gubler, P., Volkel, V., Flandin, P. and Vassalli, J.D. (1998) Masking, unmasking, and regulated polyadenylation cooperate in the translational control of a dormant mRNA in mouse ooyctes. Genes Dev., 12, 2535±2548. Thall, A.D., Maly, P. and Lowe, J.B. (1995) Oocyte gal alpha 1,3gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J. Biol. Chem., 270, 21437± 21440. Tong, Z.-B. and Nelson, L.M. (1999) A mouse gene encoding an oocyte antigen associated with autoimmune premature ovarian failure. Endocrinology, 140, 3720±3726. Tong, Z.-B., Gold, L., Pfeifer, K.E., Dorward, H., Lee, E., Bondy, C.A., Dean, J. and Nelson, L.M. (2000) Mater, a maternal effect gene required for early embryonic development in mice. Nat. Genet., 26, 267±268. Tremblay, K.D., Dunn, N.R. and Robertson, E.J. (2001) Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development, 128, 3609±3621. Verlhac, M.H., de Pennart, H., Maro, B., Cobb, M.H. and Clarke, H.J. (1993) MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev. Biol., 158, 330±340. White, T.W. and Paul, D.L. (1999) Genetic diseases and gene knockouts reveal diverse connexin functions. Annu. Rev. Physiol., 61, 283±310. Wickramasinghe, D., Ebert, K.M. and Albertini, D.F. (1991) Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev. Biol., 143, 162±172. Worrad, D.M. and Schultz, R.M. (1997) Regulation of gene expression in the preimplantation mouse embryo: temporal and spatial patterns of expression of the transcription factor Sp1. Mol. Reprod. Dev., 46, 268± 277. Worrad, D.M., Ram, P.T. and Schultz, R.M. (1994) Regulation of gene expression in the mouse oocyte and early preimplantation embryo: developmental changes in Sp1 and TATA box- binding protein, TBP. Development, 120, 2347±2357. Yan, C., Wang, P., DeMayo, J., DeMayo, F.J., Elvin, J.A., Carino, C., Prasad, S.V., Skinner, S.S., Dunbar, B.S., Dube, J.L. et al. (2001) Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol. Endocrinol., 15, 854±866. Yeom, Y.I., Fuhrmann, G., Ovitt, C.E., Brehm, A., Ohbo, K., Gross, M., Hubner, K. and Scholer, H.R. (1996) Germline regulatory element of Oct- Mouse models for human reproduction 4 speci®c for the totipotent cycle of embryonal cells. Development, 122, 881±894. Ying, Y., Qi, X. and Zhao, G.Q. (2001) Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc. Natl Acad. Sci. USA, 98, 7858±7862. Ying, Y., Liu, X.M., Marble, A., Lawson, K.A. and Zhao, G.Q. (2000) Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol. Endocrinol., 14, 1053±1063. 403