* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download E - Waterford Public Schools
Survey
Document related concepts
Transcript
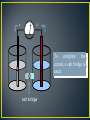
AP Chemistry • As you watch the following video, answer the questions on the sheet provided: Decoding the Past - The Real Dr. Frankenstein • Oxidation-reduction reaction (Redox) • Involves a transfer of electrons from the reducing agent to the oxidizing agent • Oxidation • Loss of electrons • LEO • OIL • Reduction • Gain of electrons • GER • RIG • • • • Oxidation number Oxidizing agent Reducing agent Half-reactions • Overall reaction is split into two half-reactions, one involving oxidation and one reduction Work on the sample Review Problem • Balance the following oxidation-reduction reaction IN BASIC SOLUTION using the half-reaction method. Be sure to identify the oxidizing agent and reducing agent. HXeO4- (s) XeO64- + Xe (g) • Luigi Galvani • Italian physician who observed a frog’s leg twitch when it was touched with two different metals • In attempting to explain what happened, Galvani thought that the animal tissue in the frog’s leg was the source of electricity • Alessandro Volta • Italian physicist who disputed Galvani’s hypothesis • Resulting controversy resulted in discovery that electric currents could be produced by chemical reactions • Volta used this discovery to create the first chemical battery • In general, all chemistry is electrical in the sense that it involves the behavior of electrons and other charged particles •The term electrochemistry is reserved specifically for the study of the interchange of chemical and electrical energy • All of the following involve the principles of electrochemistry: • Remote controls for TVs, DVD players, CD players, stereos • Itty bitty teeny tiny batteries • Calculators • Silverware • Metal-plated jewelry • Defined as a device in which chemical energy is changed to electrical energy • Examples – batteries and fuel cells • Name comes from the work of Volta and Galvani • Uses a spontaneous redox reaction to produce a current that can be used to do electrical work • Electrons flow from one terminal to the other when the terminals are connected by an external circuit 2Ag1+ + Cu(s) e- Ag 2Ag(s) + Cu 2+ e- Cu • Electrons are transferred from the ANODE to the CATHODE using an external metal conductor • Anode • Oxidation occurs here • Electrons leave the cell here Ag+ Cu2+ • Electrodes are submerged in an electrolyte • A salt solution that contains ions • Electrolyte may be involved in the reaction or the ions may be used to carry the charge • Cathode • Reduction occurs here • Electrons are accepted by the species being reduced and enter the cell here e- e- To complete the circuit, a salt bridge is used salt bridge • A salt bridge allows ion migration in solution but prevents extensive mixing of electrolytes • It neutralizes charge buildups in electrode compartments • So, it completes the circuit! • It can be a simple porous disk or a gel saturated with a non-interfering, strong electrolyte like KCl NO3NO3- is released to Cu side as Cu is converted to Cu2+ KNO3 K+ K+ is released as Ag+ is converted to Ag Salt Bridge Porous Disk Galvanic Cells • AN OX • Oxidation occurs at the anode • RED CAT • Reduction occurs at the cathode • FAT CAT • The electrons in a galvanic (voltaic) cell always flow From the Anode To the CATode • Recall that a galvanic cell consists of an oxidizing agent in one compartment that “pulls” electrons through a wire from a reducing agent in the other compartment • The “pull” or driving force on the electrons is called the CELL POTENTIAL or electromotive force (emf) • If pull occurs spontaneously, cell is a good battery! • A VOLTMETER is used to measure cell potential • The unit of electrical potential is the volt (V) • Defined as 1 joule of work per coulomb of charge transferred • The cell potential (always positive for a galvanic cell) and the balanced cell reaction is written somewhere on the diagram • The direction of electron flow is given • Obtained by inspecting the half-reactions and using the direction that gives a positive E0cell • The anode and cathode are designated • The nature of each electrode and the ions present in each compartment are labeled • A chemically inert conductor such as Pt is required if none of the substances participating in the half-reaction is a conducting solid • Example – Fe2+ and Fe3+ • Reaction in a galvanic cell is always an oxidationreduction reaction that can be broken down into two half-reactions • Each half-reaction has a cell potential • We can obtain the overall cell potential by summing the halfcell potentials! • A cell will always run spontaneously in the direction that produces a POSITIVE cell potential • Each potential is measured against an ultimate reference electrode called the STANDARD HYDROGEN ELECTRODE (SHE) • SHE consists of a piece of inert Platinum that is bathed by hydrogen gas into a 1M HCl solution at 1 atm • SHE is assigned a potential of ZERO volts Eo = 0.000 V • All other standard potentials are then reported relative to SHE H2 Pt plate 1 M HCl Reduction Half-Reaction Standard Hydrogen Electrode • Half-reaction cell potentials are listed in a convenient table! • The values in the table correspond to REDUCTION halfreactions with all solutions at 1 M, all gases at 1 atm, and 25°C (298K) for all 2+ + 2 e- → Cu Cu Symbol for standard E0 = -0.34 V versus SHE conditions! SO42- + 4 H+ + 2 e- → H2SO3 + H2O E0 = 0.20 V versus SHE • Elements that have the MOST POSITIVE reduction potentials are easily REDUCED • In general, non-metals • Elements that have the LEAST POSITIVE reduction potentials are easily OXIDIZED • In general, metals • Table can also be used to tell the strength of various oxidizing and reducing agents • Another form of the activity series • Metals having LESS POSITIVE reduction potentials are MORE active and will replace metals with more positive potentials E o, V Half reaction F2 (g) + 2e- 2F- (aq) 2.87 O2 (g) + 4H+ + 4e- 2H2O (l) 1.23 Ag+ + e- Ag (s) 0.80 Cu2+ + 2e- Cu0 0.34 2H+ + 2e- H2 (g) 0.000 Fe2+ + 2e- Fe (s) -0.44 Zn2+ + 2e- Zn (s) Al3+ + 3e- Al (s) -1.66 Li+ + e- Li (s) -3.05 -0.76 • You know that both an oxidation and a reduction must occur together • So, one of your half reactions must be reversed! • The “correct” (spontaneous or galvanic) direction for a reaction is the one where Ecell is a positive value • The half reaction with the most positive E value will proceed as a reduction! • The other will be reversed – oxidation! 1. Decide which element is oxidized or reduced using the table of reduction potentials • THE MORE POSITIVE REDUCTION POTENTIAL GETS TO BE REDUCED 2. Write both equations AS IS from the chart with their voltages 3. REVERSE the equation that will be OXIDIZED and change the sign of the voltage! • This is now E0oxidation 4. Balance the two half-reactions using integers • Number of electrons lost must equal number gained • DO NOT MULTIPLY VOLTAGE VALUES because cell potential is an intensive property! 5. Add the two half reactions and the voltages together 0 0 0 Ecell = Eoxidation + Ereduction • For our copper - zinc cell at standard conditions: E o red Cu2+ + 2eCu (s) Zn (s) Zn2+ + 2e- + 0.34 V + 0.76 V • The least positive one reverses, so the “correct” reaction at standard conditions is: Cu2+ + Zn (s) Cu (s) + Zn2+ Ecell = 0.34 v + 0.76 v = 1.10 V • Consider a galvanic cell based on the reaction: Al3+ aq + Mg s → Al s + Mg 2+ (aq) • Give the balanced cell reaction and calculate E0 for the cell • Rather than drawing an entire cell, a type of shorthand can be used – line notation • Line notation can be thought of as an “Ion Sandwich” in alphabetical order Anode metal | Anode ion || Cathode ion | Cathode metal • “|” indicates phase boundary (solid → solution or gas or solution or gas → solid) • “||” indicates salt bridge Mg s Mg 2+ aq Al3+ aq Al (s) • Phase (AND concentration if not 1M) specified in parentheses • ZnSO4 (aq, 0.5M) • A comma should be used to separate 2 components in the same phase Pt s Fe2+ aq , Fe3+ (aq) Ag + aq Ag (s) • Calculate the cell voltage for the following reaction. Draw a diagram of the galvanic cell for the reaction and label completely • See previous slide for requirements of a complete diagram Fe3+ (aq) + Cu (s) → Cu2+ (aq) + Fe2+ (aq)