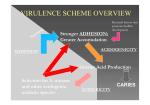
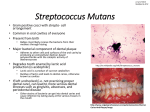
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Glucansucrases: mechanism of action and structure–function
Survey
Document related concepts
Transcript
FEMS Microbiology Reviews 23 (1999) 131^151 Glucansucrases: mechanism of action and structure^function relationships Vincent Monchois, Reneè-Marc Willemot, Pierre Monsan * Centre de Bioingeènierie Gilbert Durand, UMR CNRS 5504, LA INRA, INSA, Complexe Scienti¢que de Rangueil, 31077 Toulouse Cedex 4, France Received 21 April 1998; received in revised form 12 October 1998 ; accepted 19 November 1998 Abstract Glucansucrases are produced principally by Leuconostoc mesenteroides and oral Streptococcus species, but also by the lactic acid bacteria (Lactococci, Lactobacilli). They catalyse the synthesis of high molecular weight D-glucose polymers, named glucans, from sucrose. In the presence of efficient acceptors, they catalyse the synthesis of low molecular weight oligosaccharides. Glucosidic bond synthesis occurs without the mediation of nucleotide activated sugars and cofactors are not necessary. Glucansucrases have an industrial value because of the production of dextrans and oligosaccharides and a biological importance by their key role in the cariogenic process. They were identified more than 50 years ago. The first glucansucrase encoding gene was cloned more than 10 years ago. But the mechanism of their action remains incompletely understood. However, in order to synthesise oligosaccharides of biological interest or to develop vaccines against dental caries, elucidation of the factors determining the regiospecificity and the regioselectivity of glucansucrases is necessary. The cloning of glucansucrase encoding genes in addition to structure^function relationship studies have allowed the identification of important amino acid residues and have shown that glucansucrases are composed of two functional domains: a core region (ca. 1000 amino acids) involved in sucrose binding and splitting and a C-terminal domain (ca. 500 amino acids) composed of a series of tandem repeats involved in glucan binding. Enzymology studies have enabled different models for their action mechanism to be proposed. The use of secondary structure prediction has led to a clearer knowledge of structure^function relationships of glucansucrases. However, mainly due to the large size of these enzymes, data on the three-dimensional structure of glucansucrases (given by crystallography and modelling) remain necessary to clearly identify those features which determine function. z 1999 Federation of European Microbiological Societies. Published by Elsevier Science B.V. All rights reserved. Keywords : Dextransucrase; Glucosyltransferase ; Leuconostoc mesenteroides ; Streptococcus; Glucan binding; Glycosylhydrolase; Oligosaccharide Contents 1. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. Mechanism of glucansucrase action . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . * Corresponding author. Tel.: +33 (5) 6155-9415; Fax: +33 (5) 6155-9399; E-mail: [email protected] 0168-6445 / 99 / $20.00 ß 1999 Federation of European Microbiological Societies. Published by Elsevier Science B.V. PII: S 0 1 6 8 - 6 4 4 5 ( 9 8 ) 0 0 0 4 1 - 2 FEMSRE 642 16-4-99 132 134 132 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 2.1. Glucan biosynthesis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.1.1. Initiation of the reaction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.1.2. Elongation step . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.1.2.1. Elongation occurring at the non-reducing end of the glucan chain 2.1.2.2. Elongation occurring at the reducing end of the glucan chain . . . 2.2. Acceptor reaction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.2.1. Oligosaccharide synthesis reaction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.2.1.1. Type of acceptors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.2.1.2. Mechanism of acceptor reaction . . . . . . . . . . . . . . . . . . . . . . . . . 2.2.2. Glucan as an acceptor: mechanism proposed for branching . . . . . . . . . . . 3. Structural and functional organisation of the glucansucrases . . . . . . . . . . . . . . . . . . . 3.1. The N-terminal end of glucansucrases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.2. The presence of two functional domains . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4. Structure^function relationships of the N-terminal catalytic domain . . . . . . . . . . . . . 4.1. Identi¢cation of the catalytic site . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.2. Identi¢cation of other important regions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.3. Secondary structure prediction of the N-terminal catalytic domain . . . . . . . . . . . 5. Structure^function relationships of the C-terminal glucan binding domain . . . . . . . . . 5.1. Structure of the C-terminal domain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.2. Role of the C-terminal domain in glucan binding . . . . . . . . . . . . . . . . . . . . . . . 5.3. Role of the C-terminal domain in glucansucrase activity . . . . . . . . . . . . . . . . . . 6. Conclusions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . References ............................................................... 1. Introduction Glucansucrases (EC 2.4.5.1) are extracellular enzymes mainly produced by the soil bacterium Leuconostoc mesenteroides (commonly termed dextransucrase), Streptococcus species from the oral £ora (commonly termed glucosyltransferases) and by lactic bacteria Lactococci [1]. They catalyse the synthesis of high molecular weight D-glucose polymers named glucans from sucrose. When e¤cient acceptors, like maltose or isomaltose, are added to the reaction medium, glucansucrase catalyses the synthesis of low molecular weight oligosaccharides instead of high molecular weight glucan [2]. Di¡erent kinds of glucans with di¡erent sizes and structures, depending on the glucansucrase-producing strain, are synthesised [1,3,4]. They present a main linear chain composed of D-glucopyranosyl units, either principally linked through K(1^6) glucosidic bonds (dextran polymer), or through K(1^3) glucosidic bonds (mutan polymer) as well as linked alternately through K(1^6) and K(1^3) glucosidic bonds (alternan polymer). Glucans also di¡er in the type of branch linkages, resulting from K(1^2), K(1^3), K(1^4) and K(1^6) glucosidic bonds, the de- . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 134 135 135 135 135 137 137 137 137 138 138 138 139 139 139 140 141 145 145 145 146 146 147 gree of branching, the length of branch chains and their spatial arrangement. Dextransucrases from L. mesenteroides are used in industry. Dextran produced by L. mesenteroides NRRL B-512F was one of the ¢rst biopolymers to be produced on an industrial scale in 1948 [5] and to ¢nd several applications in medicine, separation technology and biotechnology [6,7]. Oligosaccharides produced by L. mesenteroides NRRL B-1299 with one or more D-glucopyranosyl units linked through K(1^2) glucosidic bonds [8] are highly resistant to attack by digestive enzymes [9] and are used as prebiotics in cosmetic and human nutritional applications, as they are speci¢cally metabolised by bene¢cial saprophite £ora. Glucansucrases produced by oral streptococci, like Streptococcus mutans and Steptococcus sobrinus, play a key role in the cariogenesis process, as the glucan produced enhances the attachment and colonisation of cariogenic bacteria [10,11]. In order to develop vaccines against dental caries, studies for the isolation of the genes coding for these enzymes were initiated more than 10 years ago. The sequences of 14 di¡erent glucansucrase encoding genes (named gtf) are now available [12^33] (Table 1). FEMSRE 642 16-4-99 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 133 Table 1 Main characteristics of glucansucrases from Streptococcus mutans and L. mesenteroides Strain Gene S. mutans GS5 gtf-B gtf-C gtf-D S. mutans LM7 gtf-C S. downei Mfe28 gtf-I gtf-S Glucana 87% 13% 85% 15% 30% 70% K(1^3) K(1^6) K(1^3) K(1^6) K(1^3) K(1^6) nd 88% 12% 10% 90% K(1^3) K(1^6) K(1^3) K(1^6) Size (aa) Mr b (103 ) Primerc References 1475 150 3 [12,13] 1375 140 3 [14,15] 1430 155 + [16,17] 1375 150 + [18] 1556 160 + [19^21] 1328 147 3 [19,22] S. sobrinus 6715 (serotype g) gtf-Ia nd 1592 160 + [23] S. sobrinus OMZ176 (serotype d) gtf-T 27% K(1^3) 73% K(1^6) nd 1542 163 3 [24,25] 1590 175 + [26] 90% 10% 100% 50% 50% 5% 95% K(1^3) K(1^6) K(1^6) K(1^3) K(1^6) K(1^3) K(1^6) 1522 168 + [27^29] 1599 1490 176 157 +/3 3 [27^30] [29,31,32] 1576 171 3 [29,32] gtf-Is S. salivarius ATCC 25975 gtf-J gtf-K gtf-L gtf-M S. gordonii (S. sanguis) gtf-G 40% K(1^3) 60% K(1^6) 1578 170 3 [33] L. mesenteroides NRRL B-512F dsr-S 5% K(1^3) 95% K(1^6) 1527 170 3 [34,84] L. mesenteroides NRRL B-1299 dsr-A 15% K(1^3) 85% K(1^6) 5%K(1^3) 95% K(1^6) 1290 146 3 [35] 1508 167 3 [36] dsr-B Glucan characterisations were achieved using glucansucrases produced by expression of genes cloned in E. coli. Structure of the produced glucan. nd, not determined. Percentages are percentages of total linkages in glucan structure. b Molecular weight deduced from protein sequences. c Activator e¡ect (+) or not (3) of an exogenous glucan. a Sequence information for gtf and for the more recently cloned L. mesenteroides glucansucrase encoding genes (dsr) [34^36] show that they are closely related and have a common structure. They code for large enzymes with an average molecular weight of 160 000. These proteins present an N-terminal conserved catalytic domain of about 900 aa and a C-terminal domain covering 300^400 aa composed of a series of homologous, directly repeating units and responsible for glucan binding. Some studies have allowed the precise identi¢cation of the regions essential for enzyme catalysis. However, key elements de¢ning the regioselectivity and stereospeci¢city of glucansucrases remain to a large part unknown FEMSRE 642 16-4-99 134 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 Fig. 1. Reactions catalysed by glucansucrases. I, Glucan synthesis by successive transfer of glucosyl units ; II, sucrose hydrolysis by transfer of the glucosyl unit onto water ; III, oligosaccharide synthesis by transfer of the glucosyl unit onto an acceptor molecule ; and IV, isotopic exchange by reverse reaction of glucosyl^enzyme complex formation. and, up to now, no crystallographic data are available for glucansucrases. This review is focused on the di¡erent mechanisms proposed for the di¡erent reactions catalysed by glucansucrases, as well as on the di¡erent data provided by structure^function relationship studies carried out on these enzymes by using molecular biology techniques. 2. Mechanism of glucansucrase action Glucansucrases catalyse transfer of glucosyl residues coming from sucrose cleavage. The synthesis of di¡erent products by glucansucrases depends on the destination of these glucosyl units [37] (Fig. 1). The dextran synthesis reaction occurs by successive transfer of glucosyl units to the polymer. In the presence of acceptor molecules in the reaction medium, the transfer of the glucosyl units is made onto these molecules, leading to oligosaccharide synthesis (acceptor reaction). Glucansucrases can also transfer glucosyl units onto water molecules and simply hydrolyse sucrose. Whatever the glucosyl units' destination is, their transfer supposes ¢rst the formation of a covalent glucosyl^enzyme complex [38^40] which has been isolated by Mooser and Iwaoka [41] for the glucansucrase produced by S. sobrinus. Except for the formation of the covalent glucosyl^enzyme complex, the reverse reaction being called isotopic exchange, the other reactions are mainly irreversible and are in competition with the reactive intermediary glucosyl^enzyme [42]. Sucrose is the only natural donor substrate for this synthesis [43]. Sucrose might induce a change in protein conformation that might activate the catalytic site [37]. Energy necessary to catalyse all the reactions comes only from the cleavage of the glucosidic bond of sucrose. The mediation of nucleotide-activated sugars or cofactors is not necessary. The reaction occurs with a retention of the K-con¢guration of the anomer during the cleavage of the glucosidic bond [44]. 2.1. Glucan biosynthesis Glucan synthesis occurs by autopolymerisation and is a single chain mechanism: this elongation is catalysed by a unique type of enzyme. No oligosaccharide is detected at the beginning of the reaction. Isolated glucan at a low rate of sucrose conversion, already presented a high molecular weight [45,46]. Tsuchiya et al. [46] have proposed a mechanism in which glucan chains were tightly bound with the enzyme during their elongation. It has been proposed that glucan is bound to the enzyme through noncovalent links [38]. More recently, results coming from inhibition kinetic studies using various agents suggested that the glucan binding site was separated from the sucrose binding site [47^51]. This hypothesis has also been con¢rmed by the identi¢cation of FEMSRE 642 16-4-99 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 glucansucrase sequences and by structure^function relationship studies (see Section 3). Tsuchiya et al. [46] proposed that the glucan synthesis mechanism may be separated in three di¡erent steps: (1) initiation; (2) elongation; and (3) termination. The last step corresponds to the dissociation of the glucan from the enzyme. 2.1.1. Initiation of the reaction The necessity of a primer to start the polymerisation has been often discussed. The necessity of a primer was ¢rst accepted in relation to glycogen synthesis mechanism studies carried out at the same period [43]. This hypothesis has been supported by the fact that addition of exogenous glucan has an activating e¡ect on glucan synthesis [50,52,53]. However, glucansucrases are active enzymes in the absence of any exogenous primer. Robyt and Corrigan [54] have shown that a dextran presenting blocked hydroxyl groups linked to C6 of the glucosyl residue located at its non-reducing end remained a strong activator of glucan synthesis. Exogenous glucan seems not to play the same role of primer as glycogen or starch for amylophosphorylases. Its activator e¡ect may be due to a conformational e¡ect [55]. Binding of glucan to enzyme may promote a change in the catalytic site conformation allowing the enzyme to become more active [37]. 2.1.2. Elongation step The mechanism of autopolymerisation and direction of chain growth remains an aspect still not fully understood. 2.1.2.1. Elongation occurring at the non-reducing end of the glucan chain An action mechanism similar to that of glycosidases has been proposed for glucansucrases (Fig. 2A) [37,50,56]. This mechanism involved the presence of an aspartic (or glutamic) acid acting as a nucleophilic group and another residue acting as a proton donor [57]. The carboxyl group might make a nucleophilic attack on the C1 of the glucosyl moiety of sucrose, leading to the formation of a covalent glucosyl^enzyme complex. The other acidic group might facilitate the release of fructose by giving a proton to the oxygen atom involved in the glucosidic link. It also enables another glucosyl residue to be 135 activated by trapping the hydrogen from the hydroxyl group linked to the C6 . If glucan biosynthesis follows this proposed mechanism, the result is that elongation occurs at the nonreducing end of the glucan chain. Only one covalent glucosyl^enzyme complex is then required. This mechanism has been considered as irrelevant by Su and Robyt [58] because a presence of a primer initiating the glucan synthesis reaction is required in this mechanism and, according to them, sucrose seems not to be able to participate in the chain initiation. 2.1.2.2. Elongation occurring at the reducing end of the glucan chain Ebert and Schenk [38] were the ¢rst to propose a mechanism where glucan elongation occurred at its reducing end. The glucan chain may grow by successive insertions of glucose units between the enzyme catalytic site and the reducing end of the polysaccharide. Robyt et al. [39] have shown by pulse-chase experiments using sucrose labelled with 14 C that glucan and glucosyl residues coming from sucrose cleavage were both covalently linked with enzyme through their reducing end. Similar experiments carried out with S. mutans 6715 glucansucrase [59] or with an immobilised glucansucrase free of glucan from S. sanguis ATCC 10558 [60] led to the same conclusion: chain growth occurs at the reducing end. To explain how glucan elongation occurs at the reducing end, Robyt et al. [39] have proposed a mechanism in which they suggested that two identical nucleophilic sites are involved (Fig. 2B). This mechanism is composed of two distinct steps: (1) the two nucleophilic sites attack two sucrose molecules leading to the release of two fructose molecules and giving two glucosyl residues covalently linked to the enzyme. The mechanism of the formation of these two covalent glucosyl^enzyme complexes may be identical to the action mechanism of glycosidases; and (2) the OH-C6 of one of the two glucosyl residues may make a nucleophilic attack on the C1 of the other one. That promotes the formation of an K(1^6) bond and the release of one of the two nucleophilic sites which can attack another sucrose in order to create a new covalent glucosyl^enzyme complex. FEMSRE 642 16-4-99 136 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 Fig. 2. Mechanisms of action proposed for glucansucrases. (A) Mechanism involving one nucleophilic site. (B) Mechanism involving two nucleophilic sites. X, nucleophilic group; A, proton donor group. Glucan elongation occurs by the repetition of this mechanism. The two catalytic sites are alternately involved in covalent complexes with glucosyl residue or glucan (Fig. 2B). At each step, a glucosyl residue is inserted at the reducing end of the glucanosyl^ enzyme complex. Fructose may act as an acceptor to the glucanosyl chain linked to the enzyme, result- ing in the chain termination [39]. However, up to now, the presence of fructose at the reducing end of glucan has never been reported. An interesting aspect of this mechanism is that it allows a glucan elongation by its reducing end without requiring the presence of exogenous primer at the beginning of the reaction. A kinetic study FEMSRE 642 16-4-99 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 achieved by Germaine and Schachtele [61] with glucansucrase from S. mutans 6715 concluded that two sucrose binding sites, but only one in the presence of glucan, existed in this enzyme. Another study, in which glucansucrase from S. sanguis ATCC 10558 was photolabelled using p-azidophenyl-K-D-glucopyranoside (APG), has suggested that there were two substrate binding sites [62]. E¡ect of the binding of 6-deoxysucrose, a strong inhibitor of glucansucrase, with L. mesenteroides NRRL B-512F enzyme tended also to show that two sucrose binding sites really existed [58]. However, up to now, only one site (being an aspartic acid) capable of making a covalent bond with the glucose moiety coming from the breakdown of sucrose has been clearly identi¢ed [41]. Considering the sequential insertion, it is also di¤cult to understand how the OH-C6 of one glucosyl group is able to attack the C1 of the dextransosyl group when it is far away (Fig. 2B), except if a con¢guration inversion occurs. So despite intensive work carried out for more than 25 years to elucidate the mechanism of glucansucrase action, none of the proposed mechanisms appears to be the relevant one. 2.2. Acceptor reaction The acceptor reaction was ¢rst described by Koepsell et al. [2]. In the presence of sucrose, they have observed that introduction into the reaction medium of molecules, like maltose, isomaltose, and O-Kmethylglucoside, shifted the pathway of glucan synthesis towards the production of oligosaccharides. Moreover, glucan itself can act as an acceptor molecule to yield a branched polysaccharide. 2.2.1. Oligosaccharide synthesis reaction 2.2.1.1. Type of acceptors The acceptor reaction has been studied with dextransucrases from L. mesenteroides and more particularly L. mesenteroides NRRL B-512F. Although dextransucrases accept a limited number of donor substrates other than sucrose [63^65], numerous sugars can act as acceptors [2,66]. A speci¢city of recognition seems to exist. Yamamauchi and Ohawada [67] showed that K,K-trehalose, but neither K,L-trehalose nor L,L-trehalose, acted as acceptors. More- 137 over, galactose, the limit dextrins produced by glucoamylase, or amino acid residues and peptides are not acceptors [2]. These di¡erent acceptors may be shared into two main classes, according to their ability to compete with glucan synthesis or their e¡ect on the reaction velocity: (1) the strong acceptors, like maltose or isomaltose, have an activator e¡ect on the reaction velocity and strongly inhibit the yield of glucan synthesis [66]; and (2) the weak acceptors, like fructose or melibiose, have an inhibitory e¡ect on the reaction [2]. The yield of oligosaccharides produced is low as well [66]. Some acceptors, for instance disaccharides such as maltose or oligosaccharides presenting an isomaltosyl residue at their non-reducing end, allow a series of oligosaccharides to be produced [68]. Synthesis progresses by successive transfers of glucosyl units to oligosaccharides which are alternately product and substrate. Other acceptors, like fructose [69], allow only one oligosaccharide (leucrose) to be produced in which the glucosyl residue coming from the breakdown of sucrose is added to the acceptor. The leucrose synthesis reaction, in fact, becomes important at the end of the glucan synthesis reaction when the fructose concentration is high. 2.2.1.2. Mechanism of acceptor reaction Robyt and Walseth [68] proposed that only one covalent glucosyl^enzyme complex was necessary and that acceptor molecules were incorporated at the reducing end of the glucan or the oligosaccharide produced. Oligosaccharide elongation might occur at the reducing end. According to the mechanism proposed by Robyt et al. [39] for glucan synthesis, oligosaccharides may be synthetised by a nucleophilic attack of the hydroxyl group located at the non-reducing end of the acceptor to the C1 of one of the two glucosyl residues involved in the two covalent glucosyl^enzyme complexes. Various experiments tended to show that the acceptor binding site is really unique and separated from the two active sites [58,70], but, up to now, there is no direct evidence that a separate acceptor binding exists. Moreover, according to Germaine and Schachtele [61] and Kobayashi and Matsuda [71], one of the two sucrose binding sites may also be an acceptor binding site. In DSR-S from L. mesenteroides NRRL-B 512F, change of Asp-551, being FEMSRE 642 16-4-99 138 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 the site of glucosyl^enzyme complex formation, in asparagine, resulted in a total loss of both glucan and oligosaccharide synthesis activities [84]. The activator e¡ect of maltose on the reaction velocity may be explained by a change in the limiting step occurring during the acceptor reaction. In the presence of maltose, the formation of the glucosyl^ enzyme complex becomes the limiting step instead of the polymer transfer in the case of the glucan synthesis reaction [72]. Mayer et al. [73] attributed this activator e¡ect to a change in the enzyme conformation resulting of acceptor binding with dextransucrase. The reason why weak acceptors, such as fructose, inhibit the reaction remains mostly unclear. An earlier hypothesis supported by Koepsell et al. [2] was that these acceptors inhibited sucrose breakdown. More recently, Boker et al. [74] proposed that the presence of fructose may create a steric hindrance inhibiting the growth of the glucan chain. 2.2.2. Glucan as an acceptor: mechanism proposed for branching Glucan can also be regarded as an acceptor. Mayer et al. [73] noticed an increase in the molecular weight of exogenous glucan by acceptor reaction. The ¢nal structure of the glucan produced by S. mutans 6715 necessitated the action of several glucansucrases [75]. The transfer of glucose and glucan to a glucan chain acceptor have been noticed with the dextransucrase produced by L. mesenteroides NRRL B-512F [76]. Also, the phenomenon of insolubilisation of exogenous glucan by formation of K(1^3) linkages has also been noticed with di¡erent glucansucrases [73,77^80]. As suggested by Ebert and Brosche [81] in early work, K(1^3) branch point formation may be the result of acceptor reaction. Robyt and Taniguchi [76] reported that an K(1^3) linkage was created between the anomeric carbon involved in one covalent glucosyl^enzyme complex and the OH-C3 of a glucosyl residue of the exogenous glucan. They proposed that exogenous glucan may bind in another site and one of the hydroxyl groups may exert a nucleophilic attack on the C1 involved in the glucosyl^ or glucanosyl^enzyme complex. However, this mechanism, based upon an acceptor reaction mechanism, remains insu¤cient to explain the synthesis of highly branched glucan [82]. Cote and Robyt [83] suggested that K(1^3) linkages of a highly branched glucan may be synthesised by transfer of glucosyl residues from a glucosyl^enzyme site di¡erent from the two active sites to the glucan. 3. Structural and functional organisation of the glucansucrases Studies initiated more than 10 years ago allowed the isolation and sequencing of 17 genes coding for glucansucrases produced by oral streptococci involved in the cariogenesis process, S. mutans GS5 or LM7, S. sobrinus serotype h (S. downei Mfe28), serotype g (S. sobrinus 6715), serotype d (S. sobrinus OMZ176), by S. salivarius ATCC 25975 and by L. mesenteroides NRRL B-512F and B-1299 (Table 1) [12^36]. Isolated glucansucrases are all large enzymes with an average Mr of 160 000. Streptococci glucansucrases synthesise primarily K(1^3) rich mutan polysaccharides and K(1^6) rich dextran polysaccharides [12^33]. L. mesenteroides glucansucrases produce K(1^6) rich dextrans [34^36,84]. However, genes coding for glucansucrases-producing branched glucan through K(1^2) or K(1^4) glucosidic bond or synthesising alternan have not yet been isolated. Analysis and comparison of the di¡erent protein sequences show that these enzymes are closely related and have a common structure (Fig. 3). They are composed of four distinct structural domains [30,32,33,85]. Except for DSR-A from L. mesenteroides NRRL B-1299 [35], their N-terminal end begins with a signal peptide of 32^34 aa followed by a stretch of 123^129 amino acids which is highly variable. They present a highly conserved core region of about 1000 amino acids. This domain, also named catalytic domain, is the sucrose binding domain, capable of binding and cleaving sucrose. The C-terminal end of glucansucrases covering about 500 amino acids is composed of a series of tandem repeats and constitutes the glucan binding domain. 3.1. The N-terminal end of glucansucrases Glucansucrase signal peptides are typical signal peptides of Gram-positive bacteria. They consist of a basic N-terminal part followed by a hydrophobic core region and a more polar C-terminal region [86]. FEMSRE 642 16-4-99 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 139 Fig. 3. Schematic structure of glucansucrases for which encoding genes have been cloned. A, signal peptide ; B, variable region; C, N-terminal catalytic domain; D, C-terminal glucan binding domain. The hydrophobic region is one of the rare hydrophobic regions present in glucansucrases, these proteins being overall hydrophilic in nature [13,15,17]. The main characteristic of the structure of glucansucrase signal peptides is that it is well conserved, sharing a 42% identity [36]. A lower identity level also exists with the signal peptide of a fructosyltransferase secreted by S. mutans GS-5 [87], although there is no similarity between these two groups of enzymes [17]. This shared identity between signal peptides may indicate that these enzymes use the same secretion pathway in oral streptococci and Leuconostoc mesenteroides. The non-conserved region located just downstream of the signal peptide seems to have no important role in the enzyme mechanism. Its deletion does not a¡ect the enzyme activity [23]. Moreover, DSRA, isolated from L. mesenteroides NRRL B-1299 is an active enzyme that does not possess this variable region [35]. The signi¢cance of this variable region also remains unknown. 3.2. The presence of two functional domains Ferretti et al. [21] ¢rst demonstrated that the glucan binding domain was distinct from the domain involved in sucrose splitting and that it was located at the C-terminal end. Cloning of the 3P-end of gtf-I from S. downei Mfe28 in frame fusion with lacZ allowed non-active 65-kDa protein to be expressed able to bind to Sepharose 1000. Truncation of the C-terminal end conducted to suppress glucan binding abilities of GTF-I [21]. Mooser and Wong [88] as well as Kobayashi et al. [89] have isolated by mild trypsic digestion of S. sobrinus GTF-S and GTF-I, peptides of 60.5 or 55 kDa, respectively, having the same a¤nity for glucan as native enzyme, but displaying no activity. Sequencing of peptides from GTF-I of S. sobrinus showed that it corresponded to the C-terminal part. Moreover, Abo et al. [23] have isolated a non-active 60-kDa peptide able to bind glucan and corresponding to the C-terminal end of GTF-Ia. So, the C-terminal part covering about the last 500 aa is a functional domain able to bind glucan itself. Ferretti et al. [21] suggested that the N-terminal domain was the catalytic domain. However, C-terminal truncations led to express glucansucrases unable to produce polymer [21,23,90,91]. In some cases, a sucrose cleavage activity is retained [90]. The necessary presence of at least a part of the C-terminal domain to have a glucansucrase fully active also has been reported for a cellobiohydrolase produced by Trichoderma reesei presenting a similar two domain (catalytic and binding with cellulose) structure [92]. 4. Structure^function relationships of the N-terminal catalytic domain As shown by protein sequence alignments of different glucansucrases, the N-terminal domain is highly conserved (Fig. 4). However, these sequence alignments have not allowed the identi¢cation of consensus sequences associated with a particular type of activity (e¡ect of primer addition, type of linkage produced, among others) [30,32]. This shows that the knowledge of glucansucrase primary sequences is not su¤ciently developed for investigating structure^function relationships. Moreover, due perhaps to the large size of these enzymes, no crystallographic data are available and only few structure prediction studies have been carried out [93,94]. 4.1. Identi¢cation of the catalytic site The group of Mooser has demonstrated that a site making a covalent link with the glucosyl residue FEMSRE 642 16-4-99 140 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 coming from sucrose breakdown is present inside the N-terminal domain. Mooser and Iwakoa [41] have isolated from S. sobrinus GTF-S a covalent glucosyl^enzyme complex. Mooser et al. [95] have isolated a peptide from GTF-I and GTF-S of S. sobrinus presenting the active site. The corresponding sequences were very similar and contained three aspartic acids. Mass spectrometry analysis revealed that the Asp residue inside the peptide coming from GTF-I (Asp^Ser^Ile^Arg^Val^Asp--Ala^Val^Asp) made an ester bond with the glucosyl residue. This peptide was located at 449 aa from the N-terminal end of GTF-I. Site directed mutagenesis experiments have con¢rmed the importance of this amino acid (Fig. 4). Replacement of homologous Asp residues in the GTF-B sequence (Asp-451) by Thr, Asn and Gln [96] and in the GTF-I sequence from S. downei Mfe28 (Asp-453) by Asn [94] completely suppressed the enzyme activity. Change of Asp-551 in Asn for DSR-S also abolished the activities of glucan and oligosaccharide synthesis [84]. The mutations do not a¡ect glucan binding ability of these two enzymes, GTF-B and DSR-S [84,96]. Other mutations a¡ecting Asp residues near Asp-451 from GTF-B had no signi¢cant e¡ect on enzymatic activity [96]. The functional importance of this region has also been con¢rmed by the fact that an antibody directed against a peptide of sequence homologous to this region inhibited glucansucrase activity [97^100]. With respect to an action mechanism similar to the glycosidase action mechanism, Mooser et al. [95] suggested that at least one more amino acid has to be involved in the catalytic process to facilitate the fructose release by playing the role of proton donor. The involvement of histidines in the enzymatic mechanism has been illustrated by photooxidation experiments where only histidine residues were modi¢ed [101] or by using diethylpyrocarbonate (DEP) [102,103]. Fu and Robyt [102] also concluded from inhibition curves with DEP that two histidines were involved in the action mechanism of glucansucrases. Site-directed mutagenesis experiments have enabled functionally important conserved His to be located (Fig. 4). The replacement of His-661 of DSR-S [84] and its equivalent in GTF-B (His-561) [104] to Arg led to a very weakly active enzyme. 4.2. Identi¢cation of other important regions The region preceding the active site seems to be essential for the biological activity. A genetic polymorphism among S. mutans serotype c strains a¡ecting the region extending from aa 387 to 427 in the GTF-B sequence or from aa 413 to 453 in the GTFC sequence does not induce a change in the amino acid sequence [105,106] (Fig. 4). An antibody directed against a peptide whose sequence was homologous to the 435-453 GTF-C region has an inhibitory e¡ect on GTF-C and GTF-B activity [107]. However, this inhibitory e¡ect is not observed with GTF-D where this peptide sequence is also present. Chia et al. [107] suggested that the antibody may inhibit branching or that signi¢cant conformational di¡erences may exist for this region in GTF-B or GTF-C and GTF-D. Funane et al. [103] reported the existence of a second active site for the dextransucrase produced by L. mesenteroides NRRL B-512F in a region with a sequence homologous to the 435^453 GTFC region (corresponding to the 509^527 DSR-S region). They showed that the modi¢cation of carboxylic groups with 1-ethyl-3-(3-dimethyl-amino)propylcarbdodiimide (EDC) and glycine ethyl ester (GEE), this latter compound playing the role of nucleophile, abolished glucansucrase activity. Since addition of sucrose delayed the enzyme inactivation, authors have concluded that these modi¢ed carboxyl groups may be involved in the binding with the substrate. In order to identify them, a ¢rst EDC-GEE inactivation was carried out in the presence of sucrose monocaprate followed by a second inactivation using EDC and a £uorescent nucleophilic agent ((N-1-naphtyl)ethylenediamine, EDAN) instead of GEE. A £uorescent peptide was isolated after mild trypsic digestion of the modi¢ed enzyme. Its was determined as sequence, LQEDNSNVVVEA, and shares 58% identity with a highly conserved region in glucansucrase enzymes, corresponding to the 436^447 GTF-C region and contains one Asp and two Glu residues which were suggested as providing an essential carboxyl group [103]. While it is apparent that the peptide sequence di¡ers from the highly conserved sequence identi¢ed in all glucansucrases by nucleotide sequencing, the only region of GTF sequence with FEMSRE 642 16-4-99 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 which this aligns is the conserved region corresponding to the 436^447 GTF-C region. It seems likely that the di¡erence is due to technical problems associated with peptide sequencing. The presence of functional carboxyl groups in this region has been con¢rmed by site-directed mutagenesis experiments (Fig. 4). Substitution of Asp-511 and Asp-513 of DSR-S in Asn resulted, respectively, in a complete suppression and a strong decrease in the glucan and oligosaccharide synthesis activities [84]. The substitution of the similar amino acids (Asp-411 and Asp-413) of GTF-B in Asn showed that Asp-413 was essential, but not Asp-411, a still active enzyme being obtained after mutation [104]. The two GTF-B variants exhibited Km values similar to the wild-type GTF-B, suggesting that the two Asp residues were not directly involved in the binding of sucrose [104]. In addition, Dertzbaugh and Macrina [108] have shown that an upstream region, the 342^356 GTF-B region was also important (Fig. 4). An antibody directed against a peptide homologous to this region inhibits the synthesis of both soluble and insoluble glucan. Inhibition studies of dextransucrase produced by L. mesenteroides NRRL B-512F with o-phthaldehyde (OPA) also suggested that lysines are essential for activity [109,110]. One of these essential lysines may be close of the active site [111]. Replacement of four conserved Tyr residues at position 169^172 in GTF-B suggested that this region (Fig. 4), presenting a signi¢cant homology with repeating units thought to be involved in glucan-binding, may play a role in the enzymatic mechanism [104]. Site-directed mutagenesis experiments have also allowed the identi¢cation of amino acid residues in£uencing the structure of the glucan produced. By comparing GTF-I, GTF-S, GTF-B, GTF-C and GTF-D sequences, Shimamura et al. [112] have identi¢ed positions where amino acid residues are conserved for the enzymes producing an insoluble glucan but di¡erent from the residues present in the enzymes producing a soluble glucan. Ile-448, Asp457, Asp-567, Lys-779 and Lys-1014 present in GTF-B, an enzyme-producing insoluble glucan, have been chosen to be mutated in the corresponding amino acid present in GTF-D, an enzyme producing a soluble glucan: Val-462, Asn-471, Thr-589, Gln810 and Thr-1046, respectively [112] (Fig. 4). Only 141 the mutation D567T enabled a change in the structure of the glucan produced by GTF-B to be achieved: the percentage of K(1^3) linkages is 38%, instead of 76% with the wild-type enzyme. The mutation, T589D in GTF-D, has also an e¡ect on the glucan structure, the mutated enzyme producing an insoluble one [112]. However, even though this study clearly shows the direct involvement of these residues in the glucan structure determination, the mechanism de¢ning their involvement remains to be elucidated. 4.3. Secondary structure prediction of the N-terminal catalytic domain Ferretti et al. [21] have suggested that GTF-I possesses sequences homologous with K-amylases. The degree of homology is low, but approximates to that with typical sequences of enzymes belonging to the K-amylase superfamily [93]. The N-terminal domain secondary structure prediction studies carried out by MacGregor et al. [93] and Devulapalle et al. [94] tend to show that glucansucrases possess a (K/L)8 barrel structure, like glycosidases (including K-amylase), cyclodextrin glucanotransferase (CGTase), isoamylase, and a glucan glucosidase of S. mutans [113]. This motif, ¢rst identi¢ed for triose phosphate isomerase is found in many proteins having various functions and displaying little or no homology [114]. This motif is characterised by the presence of 8 L-strands (E1^E8) located in the core of the protein alternated with 8 K-helices (H1^H8) located at the surface of the protein. These two structure prediction studies have given a similar result concerning the location of H3^E8 [93,94] (Fig. 4). The aspartic acid involved in the covalent glucosyl^enzyme complex may be located near the C-terminal end of E4. However, H1^E3 location is not identical for these two structure predictions. For MacGregor et al. [93], a circular permutation of these elements occurred in glucansucrases: the NH2 terminal helix may be the H3 element and the next elements, E1^H1^E2^H2^E3, may be far away in the sequence instead of being located at the beginning (Fig. 4). According to Devulapalle et al. [94], these ¢rst elements may be located in the N-terminal variable region of glucansucrases. However, this last hypothesis is not consistent FEMSRE 642 16-4-99 142 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 FEMSRE 642 16-4-99 143 Fig. 4. For legend please see p. 114. V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 FEMSRE 642 16-4-99 144 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 Fig. 4. Sequence alignment of the N-terminal conserved domain of glucansucrases. Alignment was made using ClustalW. Sequences shown are these from GTF-B [13], GTF-C [15], GTF-D [17], GTF-I [21], GTF-S [22], GTF-T [25], GTF-J [28], GTF-K [30], GTF-M [32], GTFG [33], DSR-S [34], DSR-A [35] and DSR-B [36]. About 10% of the residues are identical. , identical or conserved residues in all sequences in the alignment; :, conserved substitutions; W, semi-conserved substitutions ; - - -, gap in the sequence; aa-aa-aa-aa-aa, sequence of peptides used to generate antibodies [107,108]; aa, amino acid having been mutated; t, putative catalytic residues [93,94]; F, putative residues stabilising the transition state [93]; b, putative calcium binding sites [94]; W, putative chloride binding sites [94]; E, putative residues which may play a role in the binding with acceptor molecules and in the transfer of the glucosyl residue. [93]; -Ex- and -Hx-, localisation of L-strands (E) and K-helices (H) according to MacGregor et al. [93]. Dotted horizontal lines indicate that there is a sequence gap between two adjacent blocks. 6 with the fact that DSR-A is active and does not possess this region [35]. These structure predictions allow the alignment of conserved amino acid residues in GTFs with amino acid residues playing a role in action mechanism of glycosylases. It seems that Glu-475, Asp-547 and Asp-437 of GTF-S may be involved in the catalytic mechanism [93,94] (Fig. 4). Substitution of these residues in GTF-I supported this prediction, since it led to the inhibition of enzyme activity [94]. His-546 and Gln-920 of GTF-S may stabilise the transition step. Asp-440, Asn-441, Ala-476, Trp-477 and Ser-478 of GTF-S may play a role in the binding with acceptor molecules and in the transfer of the glucosyl residue [93]. The substitution of homologous Trp residue in GTF-B (Trp-491) resulted in an enzyme devoid of activity and supported the hypothesis that this residue may play a role in the action mechanism of GTFs [104]. The role of calcium binding for Asp397 and of chloride binding for Arg-435 of GTF-S have been assigned [94]. Table 2 Comparison of the pattern of repeated units composing the C-terminal domain of glucansucrases Strain Gene Repeating units References S. mutans GS5 gtf-B gtf-C gtf-D A-A-C-A-C-A-C-A-C-A-C-A-C A-A-C-A-C-A-C-A A-A-A-A-A [13] [15] [17] S. downei Mfe28 gtf-I gtf-S A-A-C-A-C-A-C-B-A-C-B-A-C A-A-C-A-C-A-C [21] [22] S. sobrinus 6715 (serotype g) gtf-Ia A-A-C-A-C-A-C-A-C-A-C [23] S. sobrinus OMZ176 (serotype d) gtf-T gtf-Is A-A-C-A-C-C-A-A-C A-A-C-A-C-A-C-A-C-A-C [25] [26] S. salivarius ATCC 25975 gtf-J gtf-K gtf-L gtf-M A-D-A-D-A-D A-D-A-A-A-D-A-D-A-D-A-D A-A-C-A-C-A-C A-C-A-C-A-A-C-A [28] [30] [32] [32] S. gordonii (S. sanguis) gtf-G A-A-C-A-C-A-C-A-C-A-C [33] L. mesenteroides NRRL B-512F dsr-S A-C-C-A-A-C [34,115] L. mesenteroides NRRL B-1299 dsr-A dsr-B A-A-A-C-A-C-A-C A-C-C-A-A-C [35] [36] Consensus sequences [21,28,85] are the following for:A repeated units, WYYFNXDGQAATGLQTIDGQTVFDDNGXQVG ; B repeated units, VNGKTYYFGSDGTAQTQANPKGQTFKDGSVLRFYNLEGQYVSGSGWY ; C repeated units, GKIFFDPDSGEVVKNRFV; and D repeated units, GGVKNADGTYSKY. FEMSRE 642 16-4-99 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 5. Structure^function relationships of the C-terminal glucan binding domain 5.1. Structure of the C-terminal domain Sequence analysis of di¡erent glucansucrases showed that these enzymes all presented a C-terminal domain composed of a series of repeated units. They have been divided into four major classes: A, B, C and D repeats [21^23,28]. The number and distribution of these repeats is speci¢c to each enzyme (Table 2). However, it appears that D repeated units are speci¢c to enzymes produced by S. salivarius ATCC 25975. A units are always present, often in an A^C pattern [85]. The C-terminal ends of DSR-S and DSR-B are characterised by the presence of small repeated units, not very homologous to each other [34,36,115]. The exact involvement of these different patterns in glucan structure determination has not been elucidated [33]. However, except for GTFL, enzymes producing an insoluble glucan possess the same pattern, A-A-C-A-C-A-C (Table 2). Similar repeated units composed of A^C motif are present in a glucan binding protein produced by S. mutans Ingbritt [116]. They can also be found in the C-terminal part of a dextranase inhibitor protein produced by S. sobrinus UAB 108 acting like a glucan binding protein [117,118]. C-Terminal domains of Clostridium di¤cile toxins A and B as well as some lytic enzymes from S. pneumoniae present similar repeated units [119,120]. The C-terminal domain of toxin A is involved in the binding with an oligosaccharide (GalK-3GalL-4GlcNac), component of the receptors of target cells [121]. Wren [119] showed that A repeats found in the C-glucan binding domain of glucansucrases and in C. di¤cile toxins always present three conserved amino acid residues: a tyrosine^phenylalanine dipeptide and a glycine located ten residues downstream. Sequence comparison enabled a consensus sequence for these repeated units to be established. According to von Eichel-Streiber et al. [122], they are composed of the same amino acid residue pattern consisting of an aromatic amino acid residue stretch, sometimes including a tyrosine, surrounded by conserved residues. The following consensus sequence has been proposed [122]: IDGYYFD+N+G. More recently, another consensus motif named YG repeat has 145 been presented [123]: NDGYYFxxxGxxHO x(G/ N)xHO HO HO . (x, non-conserved amino acid residue; HO , hydrophobic amino acid residue). It is found both within the A^D repeats and also outside them. It includes an aromatic amino acid stretch surrounded by neutral or polar amino acid residues, such as a conserved glycine residue. Its end is composed of hydrophobic residues [123]. 5.2. Role of the C-terminal domain in glucan binding The C-terminal domain is responsible for the binding of glucan. Conserved amino acid residues may be involved in binding with glucosidic units. The clustered aromatic residues (tyrosine, tryptophane and phenylalanine) may stabilise the binding between sugar and protein by interacting with the sugar unit [124]. The polar (lysine, glycine and phenylanine) or acid (aspartic acid) residues may allow the creation of hydrogen bonds with hydroxyl residues of the sugar [124]. The presence of amino acid residues, like lysine, glycine, asparagine or serine, able to introduce £exibility into the protein structure, may allow the glucosyl residue to be correctly orientated to the binding sites [125]. Singh et al. [126] showed by using di¡erent chemical inhibitors, that tyrosine, tryptophan, histidine and aspartic (or glutamic) acid residues were important for the binding abilities of glucansucrases. After the action of o-phthaldehyde (OPA) on the glucansucrase of L. mesenteroides NRRL B-512F in the presence of T-10 glucan and mild trypsic digestion, resultant peptides contained lysines which were protected from OPA because of the binding with glucan [109]. The secondary structure prediction for repeated units carried out by von Eichel-Streiber et al. [122] suggested that direct repeating units might possess the structure of a functional binding pocket. An antiserum directed against a peptide TIDGKKYFN inhibits the cytotoxic e¡ects of C. di¤cile toxin A [120]. An antiserum directed against a peptide TGAQTIKGQKLYFKANGQQVKG present in the C-terminal domain of S. downei Mfe 28 GTF-I inhibits glucansucrase activity [127]. A 17-kDa peptide coming from mild trypsic digestion of the C-terminal domain of S. sobrinus 6715 GTF-S was able to bind with glucan [128]. FEMSRE 642 16-4-99 146 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 The minimum number of these repeated units necessary to ensure glucan binding properties was investigated. For GTF-D, the loss of only one C-terminal A repeated unit or of two N-terminal A repeated units is su¤cient to suppress its binding capacity [91]. This di¡erence tends to support the idea that all the units are not required in a similar way. Moreover, it appears that the number of required units is di¡erent for enzymes producing a soluble glucan than for those producing an insoluble one, the latter enzymes appearing less sensitive to deletions. The presence of only the ¢rst two N-terminal repeated units is su¤cient for GTF-I and GTF-Ia to bind glucan [21,23,90]. Deletions of three C-terminal units (A^C) are necessary to a¡ect binding capacity of GTF-G [129]. 5.3. Role of the C-terminal domain in glucansucrase activity The presence of the C-terminal glucan binding domain seems to be necessary to keep an active enzyme [21,23,90,91,115,129]. As for glucan binding capacities, the activity of enzymes producing a soluble glucan appears to be more a¡ected by deletions of the C-terminal domain than is the activity of enzymes producing an insoluble one. For GTF-D the loss of the C-terminal A repeated unit is su¤cient to produce an activity decrease of 90% [91]. The truncation of the last 85 amino acid residues of the C-terminal domain of DSR-S resulted in only about 25% of the initial activity being retained [115]. In contrast, deletion of the three C-terminal units (A^C) of GTF-G is necessary to produce an activity decrease of 85% [129]. Only the two N-terminal A repeated units are necessary to keep GTF-I or GTF-Ia signi¢cantly active [21,23,90]. The importance of the C-terminal domain in the glucansucrase catalytic mechanism remains mostly unknown. However, the fact that with some deleted enzymes, hydrolytic activity remains, but glucan binding and synthesis properties disappear [23,90], suggests that the C-terminal domain may be important for the polymer chain growth. C-Terminal truncations of DSR-S did neither modify the Km for sucrose, nor the optimum pH and energy of activation of this enzyme both for the dextran synthesis reaction and for the oligosaccharide synthesis reaction: it is thus supposed that the C-termi- nal domain is not directly involved in the catalytic process for dextran formation, but may make release of products from the catalytic site easier [115]. The glucansucrase glucan-binding domain seems also to be required for the synthesis of low molecular weight oligosaccharides. C-Terminal truncations of DSR-S resulted in a strong decrease in oligosaccharide synthesis velocity, whatever the acceptor molecule used (maltose or fructose) [115]. Moreover, the fact that the C-terminal domain of DSR-S modulated the size of oligosaccharides produced in the presence of maltose, without a¡ecting the reaction yield and the activator e¡ect of maltose, seems to show again that its role may be to facilitate the release of products from the catalytic site without being directly involved in the catalysis [115]. The C-terminal domain seems also be involved in glucan structure determination. Deletion of the three C-terminal units (A^C) of GTF-G led to an enzyme being obtained which produced an K(1^6) linked glucan whereas wild-type GTF-G produced a glucan composed by K(1^6) and K(1^3) glucosidic linkages [129]. The in-frame fusion of the C-terminal domain of GTF-B, an enzyme producing an insoluble glucan, with the N-terminal catalytic domain of GTFD, an enzyme producing a soluble glucan, resulted in the formation of an enzyme producing an insoluble glucan [130]. However, the inverse in-frame fusion of the C-terminal domain of GTF-D with the N-terminal catalytic domain of GTF-B did not yield an enzyme producing a soluble glucan [130]. This shows that the C-terminal domain may in£uence the structure of the produced dextran, but is not the unique determinant. 6. Conclusions Glucansucrases were ¢rst identi¢ed more than 50 years ago. They are of industrial value and biologically important due to their key role in the cariogenesis process. Moreover, these enzymes are able to synthesise oligosaccharides o¡ering large scale applications in various ¢elds and particularly in health applications. Control of regioselectivity and speci¢city of these enzymes remains the major target with a view to producing oligosaccharides of structural value [131]. However, the glucansucrase action mecha- FEMSRE 642 16-4-99 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 nism is still not fully understood. Enzymology studies have enabled two general mechanisms to be proposed involving either one nucleophilic site or two nucleophilic sites. The understanding of the mechanism of glucansucrase action is complicated by the fact that many types of products of various structures can be obtained and that glucose coming from sucrose cleavage can be transferred to the reducing side of the growing polymer chain, but also to the non-reducing end of acceptor molecules. Moreover, numerous aspects of glucansucrase action mechanism, like chain termination or primer action, are still not well known. Development of molecular biology techniques has given further insight into the glucansucrase action mechanism by allowing several glucansucrase encoding genes to be cloned and structure^function relationship studies to be started. It is clearly shown that glucansucrases are large-size enzymes presenting a two domain structure: an N-terminal catalytic domain and a C-terminal glucan binding domain. A conserved Asp amino acid has been identi¢ed that makes a covalent link with the glucose moiety coming from sucrose. Site-directed mutagenesis studies combined with secondary structure prediction studies have enabled other residues important for enzyme activity to be identi¢ed, but not their exact role in the catalytic mechanism of glucansucrases. The degree of involvement of the C-terminal glucan binding domain in the action mechanism is not fully understood and amino acid residues responsible for binding with dextran have not been identi¢ed. Comparisons of glucansucrase primary sequences are not su¤cient for identifying amino acid residues which may be involved in their mechanisms. Moreover, the lack of crystallographic data does not exclude that single amino acid residue substitution may cause general conformational changes instead of simple suppression of a functional group. Thus, elucidation of the 3-dimensional structure of glucansucrase is now the major task in further work. The structure determination of enzymatic sub-domains can also be envisaged as their crystallisation should be easier. This structural information combined with modelisation of enzyme^substrate interactions and with data coming from kinetic studies obtained with di¡erent mutants, might lead to the elucidation of the glucansucrase mechanism. However, in order to validate 147 such a mechanism, further investigations, such as the crystallisation of substrate^enzyme and/or product(s)^enzyme complexes, and the elucidation of the structure of further glucansucrases, will be necessary. References [1] Sidebotham, R.L. (1974). Dextrans. Adv. Carbohydr. Chem. Biochem. 30, 371^444. [2] Koepsell, H.J., Tsuchiya, H.M., Hellman, N.N., Kasenko, A., Ho¡man, C.A., Sharpe, E.S. and Jackson, R.W. (1953) Enzymatic synthesis of dextran. Acceptor speci¢city and chain initiation. J. Biol. Chem. 200, 793^801. [3] Seymour, F.R. and Knappk, R.D. (1980) Structural analysis of dextrans from strains of Leuconostoc and related genera, that contain 3-O-K-glucosylated-D-glucopyranosyl residues at the branched points, or in consecutive, linear position. Carbohydr. Res. 81, 105^129. [4] Hare, M.D., Svensson, S. and Walker, G.J. (1978) Characterization of the extracellular, water-insoluble K-D-glucans of oral streptococci by methylation analysis, and by enzymic synthesis and degradation. Carbohydr. Res. 66, 254^264. [5] Groenwall, A.J. and Ingelman, B.J.A. (1948) Manufacture of infusion and injection £uids. U.S. Patent 2, 437^518. [6] Garvie, E.I. (1984) Separation of species of the genus Leuconostoc and di¡erentiation of the Leuconostoc from other lactic acid bacteria, In: Methods in Microbiology (Bergan, T., Ed.), Vol. 16, pp. 147^178. Academic Press, London. [7] Soetaert, W., Schwengers, D., Bucholz, K. and Vandamme, E.J. (1995) A wide range of carbohydrate modi¢cations by a single microorganism: Leuconostoc mesenteroides. In: Carbohydrate Bioengineering (Petersen, S.B., Svensson, B. and Pederson, S., Eds.), Vol. 10, pp. 351^358. Elsevier, Amsterdam. [8] Paul, F., Lopez-Munguia, A., Remaud, M., Pelenc, V. and Monsan, P. (1992) Method of the production of K-1,2 oligodextrans using Leuconostoc mesenteroides NRRL B-1299. U.S. patent 5, 141^858. [9] Djouzi, Z., Andrieux, C., Pelenc, V., Somarriba, S., Popot, F., Paul, F., Monsan, P. and Szylit, O. (1995) Degradation and fermentation of K-glucooligosaccharides by bacterial strains from human colon : in vitro and in vivo studies in gnotobiotic rats. J. Appl. Bacteriol. 79, 117^127. [10] Hamada, S. and Slade, H.D. (1980) Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44, 331^384. [11] Loesche, W.J. (1986) Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50, 353^380. [12] Aoki, H., Shiroza, T., Hayakawa, M., Sato, S. and Kuramitsu, H.K. (1986) Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect. Immun. 53, 587^594. [13] Shiroza, T., Ueda, S. and Kuramitsu, H.K. (1987) Sequence analysis of the gtfB gene from Streptococcus mutans. J. Bacteriol. 169, 4263^4270. FEMSRE 642 16-4-99 148 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 [14] Hanada, N. and Kuramitsu, H.K. (1988) Isolation and characterization of the Streptococcus mutans gtfC gene, coding for both soluble and insoluble glucan synthesis. Infect. Immun. 56, 1999^2005. [15] Ueda, S., Shiroza, T. and Kuramitsu, H.K. (1988) Sequence analysis of the gtfC gene from Streptococcus mutans GS 5. Gene 69, 101^109. [16] Hanada, N. and Kuramitsu, H.K. (1989) Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect. Immun. 57, 2079^2085. [17] Honda, O., Kato, C. and Kuramitsu, H.K. (1990) Nucleotide sequence analysis of the Streptococcus mutans gtfD gene encoding the glucosyltransferase-S enzyme. J. Gen. Microbiol. 136, 2099^2105. [18] Pucci, M.J., Jones, K.R., Kuramitsu, H.K. and Macrina, F.L. (1987) Molecular cloning and characterization of the glucosyltransferase C gene (gtfC) from Streptococcus mutans LM7. Infect. Immun. 55, 2176^2182. [19] Gilpin, M.L., Russell, R.R.B. and Morrissey, P. (1985) Cloning and expression of two Streptococcus mutans glucosyltransferases in E. coli K-12. Infect. Immun. 49, 414^416. [20] Russell, R.R.B., Gilpin, M.L., Musaka, H. and Dougan, G. (1987) Characterization of glucosyltransferase expressed from a Streptococcus sobrinus gene cloned in Escherichia coli. J. Gen. Microbiol. 133, 935^944. [21] Ferretti, J.J., Gilpin, M.L. and Russell, R.R.B. (1987) Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus Mfe28. J. Bacteriol. 169, 4271^4278. [22] Gilmore, K.S., Russell, R.R.B. and Ferretti, J.J. (1990) Analysis of the Streptococcus downei gtfS gene, which speci¢es a glucosyltransferase that synthesizes soluble glucans. Infect. Immun. 58, 2452^2458. [23] Abo, H., Matsumura, T., Kodama, T., Ohta, H., Fukui, K., Kato, K. and Kagawa, H. (1991) Peptide sequences for sucrose splitting and glucan binding within Streptococcus sobrinus glucosyltransferase (Water-Insoluble glucan synthetase) J. Bacteriol. 173, 989^996. [24] Hanada, N., Yamashita, Y., Shibata, Y., Sato, S., Katayama, T., Takehara, T. and Inoue, M. (1991) Cloning of a Streptococcus sobrinus gtf gene that encodes a glucosyltransferase which produces a high-molecular-weight water-soluble glucan. Infect. Immun. 59, 3434^3438. [25] Hanada, N., Isobe, Y., Aizawa, Y., Katayama, T., Sato, S. and Inoue, M. (1993) Nucleotide sequence analysis of the gtfT gene from Streptococcus sobrinus OMZ176. Infect Immun. 61, 2096^2103. [26] Sato, S., Masaku, I., Hanada, N., Aizawa, Y., Isobe, Y. and Isobe, Y. (1993) DNA sequence of the glucosyltransferase gene of serotype d of Streptococcus sobrinus. DNA Seq. 4, 19^27. [27] Pitty, L.J., Gi¡ard, P.M., Gilpin, M.L., Russell, R.R.B. and Jacques, N.A. (1989) Cloning and expression of glucosyltransferase activities from Streptococcus salivarius. J. Dent. Res. 68, 1681^1682. [28] Gi¡ard, P.M., Simpson, C.L., Milward, C.P. and Jacques, N.A. (1991) Molecular characterization of a cluster of at least [29] [30] [31] [32] [33] [34] [35] [36] [37] [38] [39] [40] [41] [42] [43] [44] [45] two glucosyltransferases genes in Streptococcus salivarius ATCC 25975. J. Gen. Microbiol. 137, 2577^2593. Simpson, C.L., Cheetham, N.W.H., Gi¡ard, P.M. and Jacques, N.A. (1995) Four glucosyltransferases, GTFJ, GTFK, GTFL and GTFM from Streptococcus salivarius ATCC 25975. Microbiology 141, 1451^1460. Gi¡ard, P.M., Allen, D.M., Milward, C.P., Simpson, C.L. and Jacques, N.A. (1993) Sequence of the gtfK gene of Streptococcus salivarius ATCC 25975 and evolution of the gtf genes of oral streptococci. J. Gen. Microbiol. 139, 1511^1522. Banas, J.A., Simon, D., Williams, L.K., Ferretti, J.J. and Russell, R.R.B. (1994) Analysis of a primer-independent GTF-I from Streptococcus salivarius. FEMS Microbiol. Lett. 123, 349^354. Simpson, C.L., Gi¡ard, P.M. and Jacques, N.A. (1995) Streptococcus salivarius ATCC 25975 possesses at least two genes coding for primer independent glucosyltransferases. Infect. Immun. 63, 609^621. Vickermann, M.M., Sulavik, M.C., Nowak, J.D., Gardner, N.M., Jones, C.W. and Clewell, D.B. (1997) Nucleotide sequence analysis of the Streptococcus gordonii glucosyltransferase gene, gtfG. DNA Seq. 7, 83^95. Wilke-Douglas, M., Perchorowicz, J.T., Houck, C.M. and Thomas, B.R. (1989) Methods and compositions for altering physical characteristics of fruit and fruit products. PCT patent WO 89/12386. Monchois, V., Willemot, R.M., Remaud-Simeon, M., Croux, C. and Monsan, P. (1996) Cloning and sequencing of a gene coding for a novel dextransucrase from Leuconostoc mesenteroides NRRL B-1299 synthesizing only K(1^6) and K(1^3) linkages. Gene 182, 23^32. Monchois, V., Remaud-Simeon, Monsan, P. and Willemot, R.M. (1998) Cloning and sequencing of an extracellular dextransucrase (DSRB) from Leuconostoc mesenteroides NRRL B-1299 synthesizing only K(1^6) glucan. FEMS Microbiol. Lett. 159, 307^315. Mooser, G. (1992) Glycosidases and glycosyltransferases. The Enzymes. Vol XX, pp. 187^221. Academic Press, New York. Ebert, K.H. and Schenk, G. (1968) Mechanism of biopolymer growth: the formation of dextran and levan. Adv. Enzymol. 30, 179^210. Robyt, J.F., Kimble, B.K. and Walseth, T.F. (1974) The mechanism of dextransucrase action. Direction of dextran biosynthesis. Arch. Biochem. Biophys. 165, 634^640. Luzio, G.A. and Mayer, R.M. (1983) The hydrolysis of sucrose by dextransucrase. Carbohydr. Res. 111, 311^318. Mooser, G. and Iwaoka, K.R. (1989) Sucrose 6-K-glucosyltransferase from Streptococcus sobrinus: characterization of a glucosyl^enzyme complex. Biochemistry 28, 443^449. Mayer, R.M. (1987) Dextransucrase: a glucosyltransferase from Streptococcus sanguis. Methods Enzymol. 138, 649^661. Hehre, E.J. (1941) Comparison of dextran synthesis by Leuconostoc enzyme with starch synthesis with potato phosphorylase. Proc. Soc. Exp. Biol. Med. 54, 240^241. Hestrin, S., Avireni-Shapiro, S. and Aschner, M. (1943) The enzymatic production of levan. Biochemistry 37, 450^456. Bovey, F.A. (1959) Enzymatic polymerisation I. Molecular FEMSRE 642 16-4-99 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 [46] [47] [48] [49] [50] [51] [52] [53] [54] [55] [56] [57] [58] [59] [60] [61] weight and branching during the formation of dextran. J. Polymer Sci. 35, 167^182. Tsuchiya, H.M., Hellman, N.N. and Koepsell, H.J. (1953) Factors a¡ecting molecular weight of enzymatically synthesized dextran. J. Am. Chem. Soc. 75, 757^758. Kobayashi, M., Yokoyama, I. and Matsuda, K. (1984) Activation of dextransucrase from Leuconostoc mesenteroides by the substrate dextran. Agric. Biol. Chem. 48, 221^223. Kobayashi, M., Yokoyama, I. and Matsuda, K. (1985) E¡ectors di¡erently modulating the dextransucrase activity of Leuconostoc mesenteroides. Agric. Biol. Chem. 49, 3189^3195. Kobayashi, M., Mihara, M. and Matsuda, K. (1986) Dextransucrase from Leuconostoc mesenteroides NRRL B-512F: characterization of the enzyme bound to sephadex gel. Agric. Biol. Chem. 50, 551^557. Kobayashi, M., Yokoyama, I. and Matsuda, K. (1986) Substrate binding sites of Leuconostoc dextransucrase evaluated by inhibition kinetics. Agric. Biol. Chem. 50, 2585^2590. Yokoyama, I., Kobayashi, M. and Matsuda, K. (1985) Comparison of the multplicity of dextransucrases from six strains of Leuconostoc mesenteroides. Agric. Biol. Chem. 49, 501^507. Germaine, G.R., Chludzinski, A.M. and Schachtele, C.F. (1974) Streptococcus mutans dextransucrase: requirement for primer dextran. J. Bacteriol. 120, 287^294. Germaine, G.R., Harlander, S.K., Leung, W.L.S. and Schachtele, C.F. (1977) Streptococcus mutans : functioning of primer dextran and endogenous dextranases in water-soluble and water-insoluble glucan synthesis. Infect. Immun. 16, 637^648. Robyt, J.F. and Corrigan, A.J. (1977) The mechanism of dextransucrase action. Activation of dextransucrase from Streptococcus mutans OMZ 176 by dextran and modi¢ed dextran and the non existence of the primer requirement for the synthesis of dextran. Arch. Biochem. Biophys. 183, 726^731. Robyt, J.F., Kim, D. and Yu, L. (1995) Mechanism of dextran activation of dextransucrase. Carbohydr. Res. 266, 293^ 299. Mooser, G., Shur, D., Lyou, M. and Watanabe, C. (1985) Kinetic studies on dextransucrase from the cariogenic oral bacterium Streptococcus mutans. J. Biol. Chem. 260, 6907^ 6915. Sinnot, M.L. (1990) Catalytic mechanisms of enzymic glycosyl transfer. Chem. Rev. 90, 1171^1202. Su, D.L. and Robyt, J.F. (1994) Determination of the number of sucrose and acceptor binding sites for Leuconostoc mesenteroides B-512FM dextransucrase, and the con¢rmation of the two site mechanism for dextran synthesis. Arch. Biochem. Biophys. 308, 471^476. Robyt, J.F. and Martin, P.J. (1983) Mechanism of synthesis of D-glucans by D-glucosyltransferases from Streptococcus mutans 6715. Carbohydr. Res. 113, 301^315. Ditson, S.L. and Mayer, R.M. (1984) Dextransucrase : the direction of chain growth during autopolymerisation. Carbohydr. Res. 126, 170^175. Germaine, G.R. and Schachtele, C.F. (1976) Streptococcus mutans dextransucrase : mode of interaction with high molecular-weight dextran and role in cellular aggregation. Infect. Immun. 13, 365^372. 149 [62] Koba, S.F. and Mayer, R.M. (1991) Photolabeling of dextransucrase from Streptococcus sanguis with p-azidophenyl K-Dglucopyranoside. Carbohydr. Res. 211, 317^326. [63] Mazza, J.C., Akjerman, A. and Edwards, J.R. (1975) Synthesis of K-D-glycopyranosyl K-L-sorbofuranoside and its use as a D-glucosyl donor. Carbohydr. Res. 40, 402^406. [64] Figures, W.R. and Edwards, J.R. (1976) K-D-Glucopyranosyl £uoride as a D-glucopyranosyl donor for a glycosyltransferase complex from Streptococcus mutans FA1. Carbohydr. Res. 48, 245^253. [65] Binder, T.P. and Robyt, J.F. (1983) p-Nitrophenyl K-D-glucopyranoside, a new substrate to glucansucrases. Carbohydr. Res. 124, 287^299. [66] Robyt, J.F. and Elkund, S.H. (1983) Relative, quantitative e¡ects of acceptors in the reaction of Leuconostoc mesenteroides NRRL B-512F dextransucrase. Carbohydr. Res. 68, 95^ 111. [67] Yamauchi, F. and Ohawada, Y. (1969) Synthesis of oligosaccharides by growing culture of Leuconostoc mesenteroides. Part IV. Oligosaccharide formation in the presence of various types of glucobioses as acceptors. Agric. Biol. Chem. 33, 1295^1300. [68] Robyt, J.F. and Walseth, T.F. (1978) The mechanism of acceptor reactions of Leuconostoc mesenteroides NRRL B-512F dextransucrase. Carbohydr. Res. 61, 433^435. [69] Stolada, F.H., Sharpe, E.H. and Koepsell, H.J. (1956) The preparation, properties and structure of the disaccharide leucrose. J. Am. Chem. Soc. 78, 2514^2518. [70] Tanrivseven, A. and Robyt, J.F. (1992) Inhibition of dextran synthesis by acceptor reactions of dextransucrase, and the demonstration of a separate acceptor binding site. Carbohydr. Res. 225, 321^329. [71] Kobayashi, M. and Matsuda, K. (1978) Inhibition of dextran synthesis by glucoamylase and endodextranase. Carbohydr. Res. 66, 277^288. [72] Paul, F., Oriol, E., Auriol, D. and Monsan, P. (1986) Acceptor reaction of a highly puri¢ed dextransucrase with maltose and oligosaccharides. Application to the synthesis of controlled-molecular-weight dextrans. Carbohydr. Res. 149, 433^441. [73] Mayer, R.M., Matthews, M.M., Futerman, C.L., Parnaik, V.K. and Jung, S.M. (1981) Dextransucrase : acceptor substrate reactions. Arch. Biochem. Biophys. 208, 278^287. [74] Boker, M., Jordening, H. and Buchholz, K. (1994) Kinetics of leucrose formation from sucrose by dextransucrase. Biotechnol. Bioeng. 43, 392^394. [75] Figures, W.R. and Edwards, J.R. (1981) K-D-Glucosyltransferase of Streptococcus mutans : isolation of two forms of the enzyme that bind the insoluble dextran. Carbohydr. Res. 88, 107^117. [76] Robyt, J.F. and Taniguchi, H. (1976) The mechanism of dextransucrase action. Biosynthesis of branch linkages by acceptor reactions with dextran. Arch. Biochem. Biophys. 174, 129^ 135. [77] McCabe, W.L. and Smith, E.E. (1978) The dextran acceptor reactions of dextransucrase from Streptococcus mutans K1-R. Carbohydr. Res. 63, 223^239. FEMSRE 642 16-4-99 150 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 [78] Musaka, H., Shimamura, A. and Tsumori, H. (1979) E¡ects of salts on water insoluble glucan formation by glucosyltransferase of Streptococcus mutans. Infect. Immun. 23, 564^ 570. [79] Newman, B.M., White, P., Mohan, S.B. and Cole, J.A. (1980) E¡ects of dextran and ammonium sulfate on the reaction catalysed by glucosyltransferase complex from Streptococcus mutans. J. Gen. Microbiol. 118, 353^356. [80] Sato, S., Koga, T., Yakushill, T., Nagasawa, S. and Inoue, M. (1982) E¡ect of exogenous soluble dextrans on insoluble glucan synthesis by Streptococcus mutans glucosyltransferase. Microbiology 34, 99^112. [81] Ebert, K.H. and Brosche, M. (1967) Origin of branches in native dextrans. Biopolymers 5, 423^430. [82] Kim, D. and Robyt, J.F. (1996) Dextransucrase constitutive mutants of Leuconostoc mesenteroides NRRL B-1299. Enzyme Microbiol. Technol. 17, 1050^1056. [83] Cote, G.L. and Robyt, J.F. (1984) The formation of K-D-(1^3) branch linkages by K-D-glucansucrase from S. mutans 6715 producing a soluble D-glucan. Carbohydr. Res. 127, 95^ 107. [84] Monchois, V., Remaud-Simeon, M., Russell, R.R.B., Monsan, P. and Willemot, R.M. (1997) Characterization of Leuconostoc mesenteroides NRRL B-512F dextransucrase (DSRS) and identi¢cation of amino acid residues playing a key role in enzyme activity. Appl. Microbiol. Biotechnol. 48, 465^472. [85] Russell, R.R.B. (1990) Molecular genetics of glucan metabolism in oral Streptococci. Arch. Oral. Biol. 35, 53S^58S. [86] Izard, J.W. and Kendall, D.A. (1994) Signal peptides : exquisitely designed transport promoters. Mol. Microbiol. 13, 765^ 773. [87] Shiroza, T. and Kuramitsu, H.K. (1988) Sequence analysis of the Streptococcus mutans fructosyltransferase gene and £anking regions. J. Bacteriol. 170, 4263^4270. [88] Mooser, G. and Wong, C. (1988) Isolation of glucan-binding domain of glucosyltransferase (1,6-K-glucan synthase) from Streptococcus sobrinus. Infect. Immun. 56, 880^884. [89] Kobayashi, S., Koga, K., Hayashida, O., Nakano, Y. and Hasegawa, Y. (1989) Glucan-binding domain of a glucosyltransferase from Streptococcus sobrinus: isolation of a 55-kilodalton peptide trypsin digest of glucosyltransferase prebound to insoluble glucan. Infect. Immun. 57, 2210^2213. [90] Kato, C. and Kuramitsu, H.K. (1990) Carboxy-terminal deletion analysis of the Streptococcus mutans glucosyltransferase-I enzyme. FEMS Microbiol. Lett. 72, 299^302. [91] Lis, M., Shiroza, T. and Kuramitsu, H.K. (1995) Role of the C-terminal direct repeating units of the Streptococcus mutans glucosyltransferase-S in glucan binding. Appl. Env. Microbiol. 61, 2040^2042. [92] Teeri, T.T., Koivula, A., Linder, M., Reinikainen, T., Ruohonen, L., Srisodsuk, M., Claeyssens, M. and Alwyn Jones, T. (1995) Modes of action of two Trichoderma reesei cellobiohydrolases. Progr. Biotechnol. 10, 211^224. [93] MacGregor, A.E., Jespersen, H.M. and Svensson, B. (1996) A circularly permuted K-amylase type K/L barrel structure in glucan synthesizing glucosyltransferases. FEBS Lett. 378, 263^266. [94] Devulapalle, K.S., Goodman, S.D., Gao, Q., Hemsley, A. and Mooser, G. (1997) Knowledge-base model of glucosyltransferase from the oral bacteria group of mutans streptococci. Protein Sci. 6, 2489^2493. [95] Mooser, G., Hefta, S.A., Paxton, R.J., Shively, J.E. and Lee, T.D. (1991) Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus glucosyltransferases. J. Biol. Chem. 266, 8916^8922. [96] Kato, C., Nakano, Y., Lis, M. and Kuramitsu, H.K. (1992) Molecular genetic analysis of the catalytic site of Streptococcus mutans glucosyltransferases. Biochem. Biophys. Res. Commun. 189, 1184^1188. [97] Cope, P.A. and Mooser, G. (1993) Antibodies against activesite peptides common to glucosyltransferases of mutans Streptococci. Infect. Immun. 61, 4814^4817. [98] Laloi, P., Munro, C.L., Jones, K.R. and Macrina, F.L. (1996) Immunologic characteristics of a Streptococcus mutans glucosyltransferase B sucrose binding site peptide-cholera toxin B-subunit chimeric protein. Infect. Immun. 64, 28^36. [99] Smith, D.J., Taubman, M.A., King, W.F, Eida, S., Powell, J.R. and Eastcott, J. (1994) Immunological characteristics of a synthetic peptide associated with a catalytic domain of mutans streptococcal glucosyltransferase. Infect. Immun. 62, 5470^5476. [100] Taubman, M.A., Holmberg, C.J. and Smith, D.J. (1995) Immunization of rats with synthetic peptide constructs from the glucan-binding or catalytic region of mutans streptococcal glucosyltransferases protects against dental caries. Infect. Immun. 63, 3088^3093. [101] Koga, T. and Inoue, M. (1981) Inactivation of D-glucosyltransferases from oral Streptococcus mutans and Streptococcus sanguis by photochemical oxidation. Carbohydr. Res. 93, 125^133. [102] Fu, D. and Robyt, J.F. (1988) Essential histidine residues in dextransucrase : chemical modi¢cation by diethyl pyrocarbonate and dye photo-oxidation. Carbohydr. Res. 183, 97^ 109. [103] Funane, K., Shiraiwa, M., Hashimoto, K., Ichishima, E. and Kobayashi, M. (1993) An active-site peptide containing the second essential carboxyl group of dextransucrase from Leuconostoc mesenteroides by chemical modi¢cations. Biochemistry 32, 13696^13702. [104] Tsumori, H., Minami, T. and Kuramitsu, H.K. (1997) Identi¢cation of essential amino acids in the Streptococcus mutans glucosyltransferases. J. Bacteriol. 179, 3391^3396. [105] Chia, J.S., Hsu, T.Y., Teng, L.J., Chen, J.Y., Hahn, L.J. and Yang, C.S. (1991) Glucosyltransferase gene polymorphism among Streptococcus mutans strains. Infect. Immun. 59, 1656^1660. [106] Chia, J.S., Lin, S.W., Hsu, T.Y., Chen, J.Y., Kwan, H.W. and Yang, C.S. (1993) Analysis of a DNA polymorphic region in the gtfB and gtfC genes of Streptococcus mutans. Infect. Immun. 61, 1563^1566. [107] Chia, J.S., Lin, R.H., Lin, S.W., Chen, J.Y. and Yang, C.S. (1993) Inhibition of glucosyltransferase activities of Streptococcus mutans by a monoclonal antibody to a subsequence peptide. Infect. Immun. 61, 4686^4695. FEMSRE 642 16-4-99 V. Monchois et al. / FEMS Microbiology Reviews 23 (1999) 131^151 [108] Dertzbaugh, M.T. and Macrina, F.L. (1990) Inhibition of Streptococcus mutans glucosyltransferase activity by antiserum to a subsequence peptide. Infect. Immun. 58, 1509^1513. [109] Funane, K., Arai, T., Chiba, Y., Hashimoto, K., Ichishima, E. and Kobayashi, M. (1995) Sucrose and dextran-binding sites of dextransucrase analyzed by chemical modi¢cations with o-phthalaldehyde. Oyo Tos. Kaga. 42, 27^35. [110] Goyal, A. and Katiyar, S.S. (1995) Involvement of a lysine residue in the inactivation of Leuconostoc mesenteroides NRRL B-512F dextransucrase by o-phthalaldehyde. Biochem. Mol. Biol. Int. 36, 579^585. [111] Goyal, A. and Katiyar, S.S. (1995) 2,4,6 Trinitrobenzenesulphonic acid as a probe for lysine at the active site of dextransucrase from Leuconostoc mesenteroides NRRL B-512F. Biochem. Mol. Biol. Int. 36, 639^647. [112] Shimamura, A., Nakano, Y.J., Musaka, H. and Kuramitsu, H.K. (1994) Identi¢cation of amino acid residues in Streptococcus mutans glucosyltransferases in£uencing the structure of the glucan product. J. Bacteriol. 176, 4845^4850. [113] Jespersen, H.M., MacGregor, A.E., Henrissat, B., Sierk, M.R. and Svensson, B. (1993) Starch- and glycogen-debranching and branching enzymes : prediction of structural features of the catalytic (L/K)8 -barrel domain and evolutionary relationship to other amylolytic enzymes. J. Protein Chem. 12, 791^805. [114] Farber, C.K. (1993) An K/L barrell full of evolutionary trouble. Curr. Opin. Struct. Biol. 3, 409^412. [115] Monchois, V., Reverte, A., Remaud-Simeon, H., Monsan, P. and Willemot, R.M. (1998) E¡ect of Leuconostoc mesenteroides NRRL B-512F dextransucrase carboxy-terminal deletions on dextran and oligosaccharide synthesis. Appl. Environ. Microbiol. 64, 1649. [116] Banas, J.A., Russell, R.R.B. and Ferretti, J.J. (1990) Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect. Immun. 58, 667^ 673. [117] Sun, J.W., Wanda, S.Y., Camilli, A. and Curtiss III, R. (1994) Cloning and DNA sequencing of the dextranse inhibitor gene (dei) from Streptococcus sobrinus. J. Bacteriol. 176, 7213^7222. [118] Sun, J.W., Wanda, S.Y. and Curtiss III, R. (1995) Puri¢cation, characterization and speci¢city of dextranase inhibitor (Dei) expressed from Streptococcus sobrinus UAB108 gene cloned in Escherichia coli. J. Bacteriol. 177, 1703^1711. [119] Wren, B.W. (1991) A family of clostridial and streptococcal ligand-binding proteins with conserved C-terminal repeat sequences. Mol. Microbiol. 5, 797^803. 151 [120] Wren, B.W., Russell, R.R.B. and Tabaqchali, S. (1991) Antigenic cross-reactivity and functional inhibition by antibodies to Clostridium di¤cile toxin A, Streptococcus mutans glucanbinding protein and a synthetic peptide. Infect. Immun. 59, 3151^3155. [121] Tucker, K.D. and Wilkins, T.D. (1991) Toxin A of Clostridium di¤cile binds to the human carbohydrate antigens I, X, and Y. Infect. Immun. 59, 561^564. [122] von Eichel-Streiber, C., Saueborn, M. and Kuramitsu, H.K. (1992) Evidence for a modular structure of the homologous repetitive C-terminal carbohydrate-binding sites of Clostridium di¤cile toxins and Streptococcus mutans glucosyltransferases. J. Bacteriol. 174, 6707^6710. [123] Gi¡ard, P.M. and Jacques, N.A. (1994) De¢nition of a fundamental repeating unit in streptococcal glucosyltransferase glucan-binding regions and related sequences. J. Dent. Res. 73, 1133^1141. [124] Quiocho, F.A. (1986) Carbohydrate-binding proteins : tertiary structures and protein^sugar interactions. Annu. Rev. Biochem. 55, 287^315. [125] Lemieux, R.U. (1989) The origin of the speci¢city in recognition of oligosaccharides by proteins. Chem. Soc. Rev. 18, 347^374. [126] Singh, J.S., Taylor, K.J. and Doyle, R.J. (1993) Essential amino acids involved in glucan-dependent aggregation of Streptococcus sobrinus. Carbohydr. Res. 244, 137^147. [127] Smith, D.J., Taubman, M.A., Holmberg, C.F., Eastcott, J., King, W.J. and Ali-Salaam, P. (1993) Antigenicity and immunogenicity of a synthetic peptide derived from a glucanbinding domain of mutans streptococcal glucosyltransferase. Infect. Immun. 61, 2899^2905. [128] Wong, C., Hefta, S.A., Paxton, R.J., Shively, J.E. and Mooser, G. (1990) Size and subdomain architecture of the glucan-binding domain of sucrose : 3-K-D glucosyltransferase from Streptococcus sobrinus. Infect. Immun. 58, 2165^ 2170. [129] Vickermann, M.M., Sulavik, M.C., Minick, P.E. and Clewell, D.B. (1996) Changes in the carboxy-terminal repeat region a¡ect extracellular activity and glucan products of Streptococcus gordonii glucosyltransferase. Infect. Immun. 64, 5117^5128. [130] Nakano, Y.J. and Kuramitsu, H.K. (1992) Mechanism of Streptococcus mutans glucosyltransferases : hybrid^enzyme analysis. J. Bacteriol. 174, 5639^5646. [131] Monsan, P. and Paul, F. (1995) Enzymatic synthesis of oligosaccharides. FEMS Microbiol. Rev. 16, 187. FEMSRE 642 16-4-99