* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Polysaccharides Homo- and heteroglycans
Survey
Document related concepts
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Genetic code wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Signal transduction wikipedia , lookup
Biosynthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Western blot wikipedia , lookup
Protein structure prediction wikipedia , lookup
Transcript
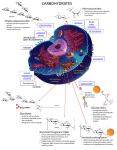
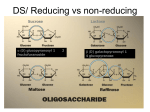
2014-10-29 Polysaccharides Homo- and heteroglycans Polysaccharides are divided into two broad classes 1. Homoglycans, or homopolysaccharides, which are polymers containing only one type of monosaccharide residue 2. Heteroglycans, or heteropolysaccharides, which are polymers containing more than one type of monosaccharide residue 1 2014-10-29 Polysaccharides - Classification Structural Polysaccharides Polysaccharides Most polysaccharides can also be classified according to their biological roles 1. Storage polysaccharides: starch and glycogen 2. Structural polysaccharides: cellulose and chitin Storage Polysaccharides 3 Starch and glycogen • Starch and glycogen are polymers of α D glucopyranose, and are known as storage polysaccharides 2 2014-10-29 Starch - a very large glucanis the plant energy reserve a. The repeating linear unit is two Glc residues in α-1→4 linkage b. The helical structure of the non-branched component amylose Amylopectin, a form of starch, have α 1-4 glycosidic bonds and α 1-6 glycosidic bonds. The actual distance between branch points averages 24 to 30 glucose residue. 3 2014-10-29 Starch digestion is sequential •α-amylose sequentially digests disaccharides from nonreducing ends of polymers; products are maltotriose, maltose, and oligosaccharides with branchpoints (dextrins) •α-glucosidase cleaves maltotriose and maltose to component glucose residues •α-dextrinase (debranching enzyme) cleaves both α-1→4 and α-1→6 linkages to degrade dextrins to monomers The major form of storage polysaccharide in animals is glycogen. • Glycogen is found mainly in the liver (where it may amount to as much as 10% of liver mass) and skeletal muscle (where it accounts for 1 to 2% of muscle mass). 4 2014-10-29 Glycogen. •Glycogen can be hydrolyzed by both α- and β-amylases, yielding glucose and maltose as products, respectively, and can also be hydrolyzed by glycogen phosphorylase, an enzyme present in liver and muscle tissue, to release glucose-1-phosphate. Cellulose • • • • Polymer of β-D-glucose attached by β(1,4) linkages Yields glucose upon complete hydrolysis Partial hydrolysis yields cellobiose Most abundant of all carbohydrates • Cotton flax: 97-99% cellulose • Wood: ~ 50% cellulose • Gives no color with iodine • Held together with lignin in woody plant tissues 5 2014-10-29 Cellulose • Cellulose monomers (βD-glucopyranose) are linked together through β1→4 glycosidic bonds by condensation. Cellulose structure•H-bonded sheets made up of linear polymer, where residues are turned 180o relative to their neighbors •H bonds are intrachain as well as interchain 6 2014-10-29 A short segment of chitin Linear polymer of N-acetylglucosamine sugars linked by β 1→4 bonds. Each GlcNAc residue is rotated 180o relative to its neighbors Heteroglycans • High molecular weight carbohydrate polymers that contain more than one kind of monosaccharides. • Major classes found in animals are N and O-linked glycans attached to proteins. 14 7 2014-10-29 Heteropolysaccharides of the animal extracellular matrix (ECM) and cell surface, termed glycosaminoglycans (GAGs), play both structural and recognition roles •Repeating disaccharides make up these polymers, and component sugars are negatively charged derivatives (uronic acids and sulfated sugars) and amino sugars (usually acetylated ) •Some GAGs have tissue-specific distribution, and all but heparin are found more often in complex structures (proteoglycans) than in isolation Structure of Glycosaminoglycans (GAGs) • Glycosaminoglycans are long, unbranched, heteropolysaccharide chains generally composed of a repeating disaccharide unit [acidic sugar–amino sugar]n. 16 8 2014-10-29 Heteroglycans, or heteropolysaccharides, • Common monosaccharide derivatives in heteropolysaccharides include: the amino sugars: D-glucosamine and D-galactosamine their derivatives: N-acetylneuraminic acid and N-acetylmuramic acid and simple sugar acids: glucuronic and iduronic acids. Some monosaccharide units found in glycosaminoglycans. 18 9 2014-10-29 Classification of the glycosaminoglycans (GAGs) • The six major classes of glycosaminoglycans are divided according to monomeric composition, type of glycosidic linkages, and degree and location of sulfate units. • The specific GAGs of physiological significance are: hyaluronic acid, dermatan sulfate, chondroitin sulfate, heparin, heparan sulfate, and keratan sulfate. 19 Highly glycosylated proteins carry up to 99% carbohydrate by weight Proteoglycans are components of the ECM, made up of GAGs, core protein, and link proteins in a complex structure •Hyaluronate forms a backbone onto which large core proteins are attached via linker proteins; other GAGs are attached to core proteins via association with protein-linked glycans. The end structures are millions of daltons, and can vary even in core and linker protein identity. •Roles of proteoglycans include both support, as described for GAGs, and recognition. Proteoglycans can bind to growth factors, allowing their presentation in the context of the ECM and limiting the target distance of the growth factors. 10 2014-10-29 Hyaluronic acid is among the largest glycans •The repeating disaccharide is Glucuronate β-1→3-GlcNAc, in β-1→4 linkages so the structure of hyaluronate is an extended helix •The numerous anionic groups result in a rigid and highly hydrated molecule- high viscosity in solution and structurally responsive to shear forces. •At low shear, high resistance as molecules are relatively disordered; at high shear, molecules line up with the direction of the force so little resistance. •This allows function as shock absorber and lubricant. Heparan SO4 is a ubiquitous cell surface and ECM GAG, and most variable in composition. The repeating disaccharide is Iduronate SO4α1→4 Sulfo-glucosamine SO4 . Heparin is similar to heparan sulfate but is less variable. This is the most highly charged GAG- there are on average 2.5 SO4 groups per disaccharide- and is the most negatively charged molecule in animal tissues. Heparin is synthesized and held within mast cells lining arteries, is released after injury and inhibits the blood clotting cascade by enhancing anti-thrombin activity. 11 2014-10-29 Heparin Heparin, a soluble glycosaminoglycan found in granules of mast cells, has a structure similar to that of heparan sulfates, but is relatively highly sulfated. made & released from mast cells in lungs & liver also associated with luminal surface of endothelium anticoagulant – forms complex with antithrombin III – this complex binds to thrombin, inactivating it – as a result, clot growth is limited – fast-acting, making it therapeutically useful Repeating units of glycosaminoglycans 12 2014-10-29 GAG Localization • Hyaluronate: synovial fluid, vitreous humor, • Chondroitin sulfate: cartilage, bone, heart valves • Heparan sulfate: basement membranes, components of cell surfaces contains higher acetylated glucosamine than heparin • Heparin: component of intracellular granules of mast cells lining the arteries of the lungs, liver • Dermatan sulfate: skin, blood vessels, heart valves• Keratan sulfate: cornea, bone, cartilage aggregated with chondroitin sulfates • Glycoconjugates = carbohydrate derivatives in which one or more carbohydrate chains are linked to a protein, peptide chain, or lipid: – Proteoglycans, peptidoglycans, glycoproteins, glycolipids 13 2014-10-29 Proteoglycans are glycosaminoglycans that are covalently linked to serine residues of specific core proteins. The linkage of GAGs to the protein core involves a specific trisaccharide composed of two galactose residues and a xylose residue (GAG-GalGalXyl-O-CH2-protein) Proteoglycans • The disaccharide units contain either of two modified sugars N-acetylgalactosamine (GalNAc) or N-acetylglucosamine (GlcNAc) and a uronic acid such as glucuronate or iduronate. 14 2014-10-29 Proteoglycan (the protein-carbohydrate) complex in cartilage A major function of GAGs is the formation of a matrix to hold together the protein components of the skin and connective tissue • In addition, carbohydrates are covalently linked with a variety of other molecules. • Carbohydrates linked to lipid molecules, or glycolipids , are common components of biological membranes. • Proteins that have covalently linked carbohydrates are called glycoproteins . 15 2014-10-29 Glycoproteins • Many proteins carry covalently attached oligosaccharide or polysaccharide chains • These complexes are known as glycoproteins, and they serve many different functions GLYCOPROTEIN FUNCTIONS • • • • • • • • • Structural→proteoglycans Transport proteins→transferrin Enzymes Antibodies Antifreeze protein in Antarctic fish -carbohydrate forms H-bonds with water, preventing growth of ice crystals Recognition phenomena-cell to molecule, cell-virus and cellcell Insulin receptor is a glycoprotein CD4 receptor is site of HIV attachment is a glycoprotein Immunoglobulins are examples of glycoproteins. 16 2014-10-29 Glycoproteins • Proteins that contain covalently-bound oligosaccharides, either to serine (O-Glycosidic linkage) or asparagine (N-glycosidic linkage) • Oligosaccharide chains exhibit great variability in sugar sequence and composition O-Glycosidic and N-glycosidic linkages Chapter 8 Mucins: salivary glycoproteins mol wt ~ 106 ~800 short (disaccharide) side chains terminal sugar is sialate – anionic sugar – at end of glycochains of many glycoproteins very hydrophilic, extended structure ~ ~ galNAc sialate – 17 2014-10-29 Mucins: modification & aggregation • • sialidase (neuraminidase) – catalyzes hydrolysis of sialates from mucins – secreted by oral bacteria products: – less hydrophilic, less H2O-soluble, more folded, more aggregated – part of the enamel pellicle & dental plaque matrix ~ ~ The blood group antigens are important group of oligosaccharides • On some cells these are attached as O-linked glycans to membrane proteins • The antigens which determine blood types belong to glycoproteins and glycolipids. 18 2014-10-29 There are three types of blood-group antigens: O, A, and B. They differ only slightly in the composition of carbohydrates • The A and B antigens are formed by addition of GalNAc or Gal, respectively to the Ooligosaccharide Peptidoglycans • The cell of many bacteria are made of peptydoglycans, which are heteroglycan chains linked to peptides • Its polysaccharide component consists of linear chains of alternating β(1→4)-linked N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (NAM) 19 2014-10-29 Glycan moiety of peptidoglycan Peptidoglycan • glycan backbone – muramic acid (NAM) – N-acetylglucosamine • peptide side chain • peptide cross-bridge – D- and L- amino acids 20 2014-10-29 Bacterial Cell Walls Composed of 1 or 2 bilayers and peptidoglycan shell • Gram-positive: One bilayer and thick peptydoglycan outer shelll Gram-positive outer shell • Gram-negative: Two bilayers with thin peptydoglycan shell in betweenhell in between • Gram-positive: pentaglycine bridge connects tetrapeptides • Gram-negative: direct amide bond between tetrapeptidesetween Gram-negative Peptidoglycan: Polymer of disaccharide N-acetylglucosamine (NAG) & N-acetylmuramic acid (NAM) Linked by polypeptides 21 2014-10-29 Peptide cross-bridges Gram-negative: Amino acids directly joined via cross-bridge Gram-positive: Glycine pentapeptide bridge joins amino acids Gram-Negative Outer Membrane Bilayer membrane outside the peptidoglycan contains phospholipids, proteins, and lipopolysaccharide (LPS) 22 2014-10-29 Lipopolysaccharide (LPS) • Lipopolysaccharide occurs on the outer membrane of gramnegative bacteria such as E. coli and Salmonella typhimurium • Also known as endotoxin • The lipid A portion of the lipopolysaccharides of some bacteria is toxic to humans (e.g. it is responsible for the dangerously lowered blood pressure that occurs with toxic shock syndrome in G(-) bacterial infections in humans – Dead cells release lipid A when cell wall disintegrates – May trigger fever, vasodilation, inflammation, shock, and blood clotting Gram-Positive cell walls • Gram-positive cell walls contain teichoic acids • Teichoic acids: – Lipoteichoic acid links to plasma membrane – Wall teichoic acid links to peptidoglycan • May regulate movement of cations • Polysaccharides provide antigenic variation 23 2014-10-29 Bacterial cell walls Teichoic acids are polymers of glycerol or ribitol (linked by phosphodiester bonds) with GlcNAc and D-Ala side chains These anionic molecules on the surface of Grampositive bacteria- up to 50% dry weight of the cell wall- can be linked to peptidoglycan at NAcGlc or NAcMur or can be linked to membrane lipid. They play structural role(s) and can be important to survival and virulence. Comparison of Gram-Positive and Gram-Negative 24