* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Arrhythmia/Electrophysiology
Survey
Document related concepts
Transcript
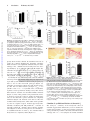
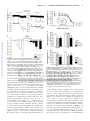
Arrhythmia/Electrophysiology Direct Inhibition of Cardiac Hyperpolarization-Activated Cyclic Nucleotide–Gated Pacemaker Channels by Clonidine Anne Knaus*; Xiangang Zong, MD*; Nadine Beetz; Roland Jahns, MD; Martin J. Lohse, MD; Martin Biel, PhD; Lutz Hein, MD Downloaded from http://circ.ahajournals.org/ by guest on June 16, 2017 Background—Inhibition of cardiac sympathetic tone represents an important strategy for treatment of cardiovascular disease, including arrhythmia, coronary heart disease, and chronic heart failure. Activation of presynaptic ␣2adrenoceptors is the most widely accepted mechanism of action of the antisympathetic drug clonidine; however, other target proteins have been postulated to contribute to the in vivo actions of clonidine. Methods and Results—To test whether clonidine elicits pharmacological effects independent of ␣2-adrenoceptors, we have generated mice with a targeted deletion of all 3 ␣2-adrenoceptor subtypes (␣2ABC⫺/⫺). ␣2ABC⫺/⫺ mice were completely unresponsive to the analgesic and hypnotic effects of clonidine; however, clonidine significantly lowered heart rate in ␣2ABC⫺/⫺ mice by up to 150 bpm. Clonidine-induced bradycardia in conscious ␣2ABC⫺/⫺ mice was 32.3% (10 g/kg) and 26.6% (100 g/kg) of the effect in wild-type mice. A similar bradycardic effect of clonidine was observed in isolated spontaneously beating right atria from ␣2ABC-knockout and wild-type mice. Clonidine inhibited the native pacemaker current (If) in isolated sinoatrial node pacemaker cells and the If-generating hyperpolarization-activated cyclic nucleotide– gated (HCN) 2 and HCN4 channels in transfected HEK293 cells. As a consequence of blocking If, clonidine reduced the slope of the diastolic depolarization and the frequency of pacemaker potentials in sinoatrial node cells from wild-type and ␣2ABC-knockout mice. Conclusions—Direct inhibition of cardiac HCN pacemaker channels contributes to the bradycardic effects of clonidine gene-targeted mice in vivo, and thus, clonidine-like drugs represent novel structures for future HCN channel inhibitors. (Circulation. 2007;115:&NA;-.) Key Words: receptors, adrenergic, alpha 䡲 heart rate 䡲 ion channels 䡲 pharmacology S tion in sympathetic tone. In addition, clonidine may activate presynaptic inhibitory ␣2-adrenoceptors on postganglionic sympathetic fibers to lower sympathetic norepinephrine release. ympathetic control of heart rate plays an important role in the pathophysiology of arrhythmias, hypertension, coronary heart disease, and chronic heart failure. At present, 3 pharmacological strategies are used in clinical medicine to reduce increased sympathetic tone, including ␣2-agonists, -adrenoceptor antagonists, and, most recently, hyperpolarization-activated cyclic nucleotide– gated (HCN) pacemaker channel inhibitors.1,2 The first antisympathetic drug established in clinical therapy was clonidine (for reviews, see Schmitt3 and Hoefke and Kobinger4). Investigation into the mechanism of action of clonidine led to the identification of ␣2-adrenoceptors as the main target of the action of clonidine.5 Despite the fact that clonidine has vasoconstrictive properties, it was introduced into clinical practice as an antihypertensive and antisympathetic drug. Clonidine may act at 2 anatomic sites to lower blood pressure.6 In several brain stem nuclei, activation of ␣2-adrenoceptors leads to a reduc- Clinical Perspective p ●●● Later, pharmacological ligands were applied to identify subtypes of ␣2-receptors, which were confirmed by molecular cloning of 3 independent ␣2-adrenoceptor genes from different species (␣2A, ␣2B, and ␣2C).7 The physiological significance of the 3 ␣2-adrenoceptor subtypes was then highlighted by targeted deletions in the murine genes.8 With these gene-targeted mouse models, the 2 major actions of clonidine and other ␣2-agonists, hypotension and sedation, could be assigned to activation of ␣2A-receptors, whereas ␣2B-receptors were involved in vasoconstriction, and ␣2C took part in modulation of catecholamine release.9 –11 However, several Received October 1, 2006; accepted December 15, 2006. From the Department of Pharmacology and Toxicology (A.K., R.J., M.J.L.) and Department of Internal Medicine (R.J.), University of Würzburg, Würzburg, Germany; Department of Pharmacy–Center for Drug Research (X.Z., M.B.), Ludwig Maximilian University of Munich, München, Germany; and Institute of Experimental and Clinical Pharmacology and Toxicology (A.K., N.B., L.H.), University of Freiburg, Freiburg, Germany. The online-only Data Supplement, consisting of a table and figures, is available with this article at http://circ.ahajournals.org/cgi/content/ full/CIRCULATIONAHA.106.667675/DC1. *The first 2 authors contributed equally to this work. Correspondence to Lutz Hein, MD, Institute of Experimental and Clinical Pharmacology and Toxicology, University of Freiburg, Albertstrasse 25, 79104 Freiburg, Germany. E-mail [email protected] © 2007 American Heart Association, Inc. Circulation is available at http://www.circulationaha.org DOI: 10.1161/CIRCULATIONAHA.106.667675 1 2 Circulation February 20, 2007 Downloaded from http://circ.ahajournals.org/ by guest on June 16, 2017 reports had suggested that not all of the effects of clonidine were dependent on ␣2-adrenoceptors, which led to the development of the “imidazoline receptor hypothesis.”12 Several different imidazoline binding sites were proposed (for review, see Szabo6); however, the molecular identity of the putative I1 imidazoline receptor that was suggested to be responsible for the hypotensive effect of clonidine and other imidazolines has not yet been uncovered. To search for non–␣2-adrenoceptor effects of clonidine, we have generated mice lacking all 3 ␣2-adrenoceptors (␣2ABC⫺/⫺). This led to the identification of a direct bradycardic effect of clonidine by inhibition of the cardiac hyperpolarization-activated (“pacemaker”) current (If). If has been shown to play a key role in the generation of pacemaker potentials in sinoatrial node (SAN) cells of the heart.1,2,13 Moreover, If is enhanced by direct interaction with cyclic adenosine monophosphate and, hence, contributes to the autonomous regulation of heart rate by the sympathetic and parasympathetic nervous system. If is encoded by a family of 4 HCN channels (HCN1–4).14 In mouse SAN, HCN4 and HCN2 are the predominantly expressed HCN channel isoforms.15–17 The same isoforms have been also detected in human heart tissue.18 Mouse SAN does not express substantial levels of HCN1, but higher levels of this subunit (⬇20% of total HCN mRNA) were found in rabbit SAN.15 Here, we show that clonidine blocks both HCN2 and HCN4 channels in the low micromolar concentration range and, as a consequence, lowers the frequency of pacemaker potentials. Methods Generation of ␣2ABC ⴚ/ⴚ Mice The generation of ␣2ABC⫺/⫺ has been described previously.19 From the initial intercrossing of Adra2a⫺/⫺Adra2b⫹/⫺Adra2c⫺/⫺ mice, a small percentage survived a defect in placental development. These mice were used to establish an independent colony of ␣2ABC-deficient mice. Mice were maintained in a specified pathogen-free facility. All animal procedures were approved by the Universities of Freiburg and Würzburg. Autoradiography and Radioligand Binding Mouse brain membranes20 were incubated in binding buffer containing (in mmol/L): 25 glycylglycine, 40 HEPES (pH 8), 5 EGTA, 5 MgCl2, 100 NaCl, 8 [3H](1,4-benzodioxan-2-methoxy-2-yl)2imidazoline hydrochloride (RX821002). Nonspecific binding was determined in the presence of 1 mol/L atipamezole. For receptor autoradiography, transverse cryostat sections of the brain (10 m) were incubated for 60 minutes in 50 mmol/L Tris-HCl (pH 7.5), 1.5 mmol/L EDTA, and 8 nmol/L [3H]RX821002. Slides were exposed to 3H-Hyperfilm (Amersham Pharmacia, Freiburg, Germany) for 16 to 24 weeks. Norepinephrine Release [3H]norepinephrine release was determined in cardiac atria essentially as described previously with minor modifications.11,20 [3H]norepinephrine release was evoked by short trains of rectangular electrical pulses (4 pulses, 100 Hz). The amount of radioactivity released from the tissues was determined by liquid scintillation counting.11 Twenty-Four–Hour Urinary Catecholamine Determination Catecholamine excretion was quantified by high-performance liquid chromatography combined with electrochemical detection of urine samples collected over 24-hour periods in metabolic cages as described previously.21 Sedation and Analgesia Fifteen minutes after clonidine injection (1 mg/kg IP), mice were placed 3 times on a rotating wheel (rotating speed 10 rpm); maximal cutoff time was 60 seconds. For analgesia testing, a tail-flick assay system (Ugo Basile, Comerio, Italy), equipped with an infrared light source and automatic recording of the reaction time, was used. Hemodynamic Measurements For cardiac, aortic, or femoral artery catheterization with a 1.4F Millar microtip device, mice were anesthetized by isoflurane (2 vol% in O2) applied by face mask and kept on a heating table at 37°C.22 Hemodynamic parameters were digitized via a MacLab system (AD Instruments, Castle Hill, Australia). Transthoracic echocardiography Doppler examinations were performed in lightly sedated (200 L 2.5% tribromoethanol IP) mice with an echocardiographic system (Acuson Sequoia C512, Siemens AG, Erlangen, Germany) equipped with a 15-MHz linear transducer (Acuson, 15L8). For measurements in conscious, unrestrained mice, blood pressure and ECGs were recorded by telemetry (DSI, Transoma Medical, St. Paul, Minn; PA10 for aortic pressure, TC10 for ECG) 10 to 20 days after surgery. Histology Hearts were fixed with 4% paraformaldehyde in phosphate-buffered saline (pH 7.4), embedded in paraffin, and stained with hematoxylineosin. Left ventricular myocyte cross-sectional areas were analyzed by computer-assisted morphometry. To detect interstitial fibrosis, hearts were stained with Sirius red as described previously.22 Organ Bath Experiments Hearts were rapidly excised and placed in carbogenated modified Tyrode’s solution (concentrations in mmol/L: 119 NaCl, 5.4 KCl, 1.4 CaCl2, 1 MgCl2, 22.6 NaHCO3, 0,42 NaH2PO4, 0.025 EDTA, 10 glucose, 0.2 ascorbic acid, pH 7.4). Right atria of 3- to 4-month-old mice were mounted in an organ bath chamber and were allowed to contract spontaneously. ␣2ABC-Knockout (KO) mice were injected 16 hour antemortem with pertussis toxin 150 g/kg IP (Sigma, Munich, Germany).23 Cell Culture and Isolation of Murine SAN Cells Human embryonic kidney (HEK)-293 cell lines stably expressing either murine HCN2 or human HCN4 were maintained as described previously.18,24 SAN cells were isolated from 6- to 12-week-old adult ␣2ABC⫹/⫹ and ␣2ABC⫺/⫺ mice of either sex by standard procedures.25,26 Electrophysiological Recordings Native If and heterologously expressed HCN channels were measured at room temperature with the whole-cell voltage-clamp technique as described previously.25 The extracellular solution was composed of (in mmol/L): 135 NaCl, 5 KCl, 1.8 CaCl2, 0.5 MgCl2, 5 HEPES, pH 7.4. For recordings of If in SAN cells, 1 mmol/L BaCl2 and 2 mmol/L MnCl2 were added to the extracellular solution. The intracellular solution contained (in mmol/L): 130 KCl, 10 NaCl, 0.5 MgCl2, 1 EGTA, 5 HEPES, pH 7.4. Spontaneous action potentials of isolated SAN cells were recorded at 30°C with the perforated patch technique with 120 g/mL amphotericin B. Effects of clonidine were determined with a repetitive stimulation protocol. Hyperpolarizing pulses of 1.0-second duration (for HCN2, HCN1, and native If; test potential ⫺100 mV for HCN2 and native If and ⫺90 mV for HCN1) or 1.5-second duration (for HCN4, test potential ⫺110 mV) were applied from a holding potential of ⫺40 mV every 2 seconds (HCN2, HCN1, and native If) or every 3 seconds (HCN4), and the resulting inward currents were determined. The longer pulse duration for HCN4 was chosen with respect to the slow activation kinetics of this channel. For determination of dose-response relationships, the maximum inward current corrected for the instantaneous current component of If27 was obtained after repetitive stimulation for 1 minute. IC50 values and Hill coefficients () were calculated by fitting with the Hill equation. Steady-state activation curves were determined by hyperpolarizing voltages of ⫺140 to ⫺30 mV from a holding potential of ⫺40 mV for 2.4 seconds followed by a step to Knaus et al Clonidine Inhibits HCN Pacemaker Channels 3 ⫺140 mV. Tail currents, measured immediately after the final step to ⫺140 mV, were normalized by the maximal current (Imax) and plotted as a function of the preceding membrane potential. The data points were fitted with the Boltzmann function: (I-I min )/ (Imax⫺Imin)⫽{1⫺exp[(Vm⫺V0.5)/k]} where Imin is an offset caused by a nonzero holding current, Vm is the test potential, V0.5 is the membrane potential for half-maximal activation, and k is the slope factor. Statistical Analysis Data were analyzed by ANOVA followed by appropriate post hoc tests, by Student t test for unpaired samples, by paired-samples t test, or by repeated-measures test when appropriate. A probability value of ⬍0.05 was considered statistically significant. Results are displayed as mean⫾SEM. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written. Results Downloaded from http://circ.ahajournals.org/ by guest on June 16, 2017 Generation of Mice Deficient in ␣2ABC-Adrenoceptors Mice lacking all 3 ␣2-adrenoceptor subtypes (␣2ABC⫺/⫺) were derived from matings of male and female Adra2a⫺/⫺ Adra2b⫹/⫺Adra2c⫺/⫺mice.19 Initially, a high percentage of ␣2ABC⫺/⫺ mice died during embryonic development due to a defect in placental vascular development19; however, from surviving ␣2ABC⫺/⫺ mice, a breeding colony could be established (Figure 1). Several methods were applied to document the deletion of all 3 ␣2-adrenoceptor genes. Autoradiography with the ␣2-receptor antagonist [3H]RX821002 revealed a high density of ␣2-receptor binding sites in wild-type brain. In the presence of the specific ␣2-adrenoceptor antagonist atipamezole, this signal was absent in ␣2ABC⫹/⫹ brains and was also undetectable in brain sections of ␣2ABC⫺/⫺ mice (Figure 1b). Similarly, quantitative radioligand binding did not detect any specific ␣2-adrenoceptors in brain membranes of ␣2ABC⫺/⫺ mice (Figure 1c). To further verify the complete deletion of the 3 ␣2adrenoceptor genes, pharmacological tests for typical ␣2receptor functions were performed. Intraperitoneal injection of the ␣2-agonist clonidine resulted in a shortened latency time on a rotating wheel, which demonstrates its sedating effect in ␣2ABC⫹/⫹ mice (Figure 2a). In contrast, clonidine did not induce sedation in ␣2ABC⫺/⫺ mice (Figure 2a). Similarly, the analgesic properties of clonidine could be verified by an increased latency time in the tail-flick assay in wild-type mice but not in ␣2ABC⫺/⫺ mice (Figure 2b). Another important function of ␣2-adrenoceptors is their role in presynaptic feedback inhibition of neurotransmitter release. In isolated heart atria from wild-type mice, norepinephrine (Figure 2c) and the ␣2-agonist medetomidine (not shown) inhibited the electrically evoked release of [3H]norepinephrine in a concentration-dependent manner. In ␣2ABC⫺/⫺ atria, norepinephrine did not inhibit sympathetic transmitter release. Genetic disruption of presynaptic feedback inhibition also resulted in enhanced sympathetic neurotransmitter release in vivo, as evidenced by increased norepinephrine excretion in 24-hour urine samples of ␣2ABC⫺/⫺ compared with wild-type mice (Figure 2d). Figure 1. Generation of mice deficient in ␣2A-, ␣2B-, and ␣2Cadrenoceptors. a, Targeted alleles of the murine ␣2A (Adra2a), ␣2B (Adra2b), and ␣2C (Adra2c) receptor genes. Primers for genotyping (right) are depicted as arrows. AWTF indicates forward primer for ␣2A wild-type allele; AWTR, reverse primer for ␣2A wild-type allele (similar for ␣2B and ␣2C alleles); and NEO, neomycin resistance cassette. b, Autoradiography of brain slices with the ␣2-adrenoceptor antagonist [3H]RX821002 revealed dense labeling in ␣2ABC⫹/⫹ cortex and hippocampus. No specific signal could be detected in the presence of the ␣2-adrenoceptor antagonist atipamezole (1 mol/L) or in brain sections from ␣2ABC⫺/⫺ mice. c, ␣2-Receptor density in brain membranes as determined by radioligand binding with [3H]RX821002 (8 nmol/ L). In contrast to wild-type brain, no specific binding signal could be detected in the presence of atipamezole or in brain membranes from ␣2ABC⫺/⫺ mice (n⫽3 experiments). Cardiovascular Function in ␣2ABC-Deficient Mice To determine the long-term consequences of enhanced sympathetic tone, we first assessed cardiovascular function in conscious, freely-moving ␣2ABC⫺/⫺ mice by telemetry (Figures 3a through 3c). Enhanced sympathetic norepinephrine release in ␣2ABC⫺/⫺ mice was accompanied by increased systolic and diastolic blood pressures and elevated heart rate (Figures 3a through 3c). At the age of 6 months, however, cardiac function was already compromised. Left ventricular fractional shortening was reduced to 33% in ␣2ABC⫺/⫺ animals compared with 50% in wild-type mice (Figure 3d). In addition, severe cardiac fibrosis and hypertrophy were detected in left ventricles of ␣2ABC⫺/⫺ hearts (Figures 3e through 3g). Next, we assessed the effects of the ␣2-agonist clonidine on blood pressure and heart rate. In wild-type mice, clonidine significantly reduced mean arterial pressure and heart rate during isoflurane anesthesia (Figure 4a). Surprisingly, the bradycardic effect of clonidine was still present in ␣2ABC⫺/⫺ mice (Figure 4a), whereas its hypotensive effect was com- 4 Circulation February 20, 2007 Downloaded from http://circ.ahajournals.org/ by guest on June 16, 2017 Figure 2. Clonidine-induced sedation, analgesia, and presynaptic feedback inhibition are absent in ␣2ABC⫺/⫺ mice. a, Clonidine (1 mg/kg IP) significantly reduced the latency time on a rotarod in wild-type but not in ␣2ABC⫺/⫺ mice. *P⬍0.05, paired t test, n⫽7 mice per genotype. b, Clonidine (1 mg/kg IP) did not induce analgesia in the tail-flick test in ␣2ABC⫺/⫺ mice. *P⬍0.01, paired t test, n⫽7 mice per genotype. c, Exogenously added norepinephrine inhibited electrically evoked release of [3H]norepinephrine in wild-type atria but not in ␣2ABC⫺/⫺ atria (n⫽6 experiments). d, Twenty-four-hour urinary excretion of norepinephrine was significantly elevated in ␣2ABC⫺/⫺ mice compared with wild-type mice (␣2ABC⫺/⫺ n⫽8, ␣2ABC⫹/⫹ n⫽10 male mice). *P⬍0.05. pletely absent in these animals. To determine heart rate in awake mice without disrupting the baroreflex, telemetric ECG transducers were implanted subcutaneously. In awake wild-type mice, clonidine lowered resting heart rate dosedependently by up to 250 bpm (300 g/kg IP; Figure 4b). Clonidine also lowered heart rate in ␣2ABC-deficient mice. Its effect in ␣2ABC⫺/⫺ mice reached 32.3%, 26.6%, and 42.6% of the bradycardia observed in wild-type mice at 10, 100, and 300 g/kg IP, respectively (Figure 4b). To identify the site of the bradycardic action of clonidine, isolated spontaneously beating right atria were tested in an organ bath system. Clonidine reduced beating frequency by 20% to 25% in atria from both genotypes (Figure 5a). Clonidine concentrations reducing the right atrial frequency by 50% did not differ between genotypes (␣2ABC⫹/⫹ 4.9 mol/L versus ␣2ABC⫺/⫺ 4.4 mol/L). The concentrations required to lower spontaneous beating rate by 20% did not differ between clonidine and the HCN inhibitor ZD7288 (Figure 5a, inset). After pertussis toxin pretreatment of mice to inactivate G proteins of the Gi/o family, the bradycardic effect of the muscarinic agonist carbachol was completely eliminated (Figure 5b). Pertussis toxin did not affect the clonidine-induced bradycardia, which ruled out the possibility that Gi/o-coupled receptors mediated the bradycardia. Similarly, incubation with 100 mol/L Ba2⫹, a blocker of inwardly rectifying K⫹ channels, did not alter the clonidine effect (Figure 5c); however, in the presence of 2 mmol/L Cs⫹, an established blocker of cardiac If, the clonidine-induced bradycardia was completely absent (Figure 5c). Figure 3. Cardiovascular function and structure of ␣2ABCdeficient mice. a, b, and c, Baseline hemodynamic parameters were assessed in awake, unrestrained mice by pressure telemetry. Mean 24-hour systolic and diastolic blood pressure and heart rate were significantly higher in ␣2ABC⫺/⫺ mice than in wildtype mice (n⫽6 mice per genotype). d, Cardiac contractile performance was determined by echocardiography under anesthesia with tribromoethanol. Left ventricular fractional shortening was reduced in ␣2ABC⫺/⫺ mice compared with wild-type controls (*P⬍0.05; n⫽4 mice per genotype). e, Areas of interstitial fibrosis are visible in the left ventricular wall of ␣2ABC⫺/⫺ but not in ␣2ABC⫹/⫹ hearts by Sirius red staining (male mice, age 6 to 9 months). Bar⫽50 m. f and g, Cardiac hypertrophy in ␣2ABC⫺/⫺ can be detected as an increased heart weight/body weight ratio (f, ␣2ABC⫹/⫹ n⫽32, ␣2ABC⫺/⫺ n⫽66 mice), as well as increased cross-sectional area of cardiac myocytes (g, n⫽6 hearts per genotype, *P⬍0.05). Clonidine Is an Efficient Blocker of Sinoatrial If The strong Cs⫹ sensitivity of the bradycardic effect of clonidine suggested that clonidine may act via If channels. To explore this hypothesis, we first characterized spontaneous action potentials of pacemaker cells isolated from the SAN of wild-type and ␣2ABC⫺/⫺ mice (Figures 6a and 6b). Clonidine Knaus et al Downloaded from http://circ.ahajournals.org/ by guest on June 16, 2017 Figure 4. Clonidine-induced bradycardia does not require ␣2-adrenoceptors. a, Hemodynamic responses to intravenous injection of clonidine were determined by femoral artery catheterization with a 1.4F microtip catheter during isoflurane anesthesia (2 vol% in O2). Original trace recordings of aortic pressure (upper traces) and heart rate (lower traces) in male ␣2ABC⫹/⫹ and ␣2ABC⫺/⫺ mice reveal bradycardic effects of clonidine in both mouse strains without significant changes in blood pressure in ␣2ABC⫺/⫺ mice. b, Bradycardic effect of clonidine in awake, freely moving mice with implantation of a telemetric ECG transducer. Clonidine lowered heart rate in conscious animals of both genotypes (n⫽6 to 9 mice per genotype, repeated-measures test, *P⬍0.05 drug vs untreated). n.d. indicates not determined. profoundly lowered the frequency of pacemaker potentials in SAN cells from both genotypes. Clonidine increased the duration between 2 peaks (cycle length) from 455⫾48 to 1105⫾223 ms (n⫽6) in wild-type mice and from 605⫾45 to 895⫾98 ms (n⫽4) in ␣2ABC⫺/⫺ mice (Figures 6a and 6b). Although the increase in cycle length induced by clonidine was highly significant in both genotypes, the absolute values of cycle length in the absence and presence of clonidine, respectively, were not statistically different between genotypes. The frequency reduction of pacemaker potentials by clonidine was accompanied by a reduction of the slope of the diastolic depolarization (wild-type: from 79.7⫾8.1 to 45.6⫾6.6 mV/s, n⫽6, P⬍0.05; ␣2ABC⫺/⫺: from 62.1⫾9.9 to 34.1⫾7.9 mV/s, n⫽4, P⬍0.05). In contrast, other parameters of the pacemaker potential (maximum diastolic potential, overshoot, and action potential duration) were unaffected by clonidine (Data Supplement, Table I). The reduction of the slope of diastolic depolarization could be caused by an inhibition of the If current. Indeed, clonidine efficiently blocked this current in a dose-dependent manner (Figures 6c Clonidine Inhibits HCN Pacemaker Channels 5 Figure 5. Bradycardic effects of clonidine in isolated, spontaneously beating right atria. a, Clonidine lowered spontaneous frequency in right atria of both genotypes with similar potency and efficacy (EC50 ␣2ABC⫹/⫹ 4.9 mol/L vs ␣2ABC⫺/⫺ 4.4 mol/L). Inset, Inhibition of spontaneous beating frequency of ␣2ABC⫺/⫺ atria by the HCN channel inhibitor ZD7288. b, The bradycardic effect of carbachol could be eliminated by overnight pretreatment of mice with pertussis toxin (PTX) to block signaling via Gi/ocoupled receptors. The bradycardic effect of clonidine was not affected by pertussis toxin. c, 100 mol/L Ba2⫹ (to block inwardly rectifying K⫹ channels) did not affect the bradycardic effect of clonidine, but 2 mmol/L Cs⫹ (to inhibit HCN channels) disrupted the bradycardia (n⫽2 atria per genotype in 3 to 4 independent experiments; b and c, repeated-measures test, *P⬍0.05 vs control, #P⬍0.01 vs Ba2⫹/Cs⫹). and 6d). The IC50 values at ⫺100 mV were 3.1⫾0.5 mol/L (n⫽5 to 11) and 2.8⫾0.7 mol/L (n⫽5 to 8) for wild-type and ␣2ABC⫺/⫺ mice, respectively, which is in excellent agreement with the IC50 values determined in beating right atria (Figure 5a). If had the same amplitude in wild-type and ␣2ABC⫺/⫺ cells (⫺5.3⫾0.8 versus ⫺6.1⫾1 pA/pF, n⫽13, P⫽0.2). Moreover, kinetics and voltage-dependence of If were indistinguishable between genotypes (data not shown). Cardiac If is mediated by HCN4 and HCN2 channels; therefore, we tested the effect of clonidine on HEK293 cell lines that stably expressed either or both channels (Figures 7a and 7b). Clonidine inhibited both channels in a dosedependent manner. The IC50 values were slightly higher than those of native If (9.8⫾1.4 mol/L [n⫽7 to 12] for HCN4 and 6 Circulation February 20, 2007 Downloaded from http://circ.ahajournals.org/ by guest on June 16, 2017 Figure 6. Clonidine reduces the frequency of spontaneous SAN pacemaker potentials by blocking If. a and b, Action potentials of SAN pacemaker cells from ␣2ABC⫹/⫹ (a) and ␣2ABC⫺/⫺ (b) mice under control conditions (black) and in the presence (red) of 10 mol/L extracellular clonidine. c, If current traces from an ␣2ABC⫺/⫺ SAN cell in the presence of clonidine as indicated. Currents were evoked by stepping from a holding potential of ⫺40 to ⫺100 mV. d, Doseresponse relationships for inhibition of If by clonidine in SAN cells of ␣2ABC⫹/⫹ (open symbols) and ␣2ABC⫺/⫺ mice (closed symbols). Solid lines are the fits to the Hill equation with the following parameters: ␣2ABC⫹/⫹: IC50⫽3.18 mol/L, ⫽0.56; ␣2ABC⫺/⫺: IC50⫽2.67 mol/L, ⫽0.56. The number of experiments for each concentration is given in parentheses. 8.2⫾1.4 mol/L [n⫽8 to 10] for HCN2; Figures 7c and 7d). Interestingly, in the presence of 100 mol/L Cs⫹, which corresponds to the half-maximal inhibitory concentration of this cation, the clonidine binding curve for HCN2 was shifted to the right (IC50⫽16.3⫾1.4 mol/L; n⫽12). This finding suggested that Cs⫹ and clonidine may competitively bind to the same channel region. Clonidine not only blocked If, it also shifted the voltage-dependence of channel activation by 10 to 20 mV to more hyperpolarizing potentials (Figures 8a through 8d). Clonidine also inhibited HCN1 currents, although with significantly lower sensitivity (IC50⫽40.1⫾4.34 mol/L; n⫽7 to 9). By contrast, clonidine had virtually no effect on voltage-gated calcium and sodium channels (Data Supplement, Figure I). Discussion Figure 7. Inhibition of heterologously expressed HCN4 and HCN2 channels by clonidine. a and b, Current traces of stably expressed HCN4 (a) and HCN2 (b) in the presence of increasing concentrations of clonidine as indicated. Currents were evoked by stepping from ⫺40 to either ⫺110mV (for HCN4) or ⫺100 mV (for HCN2). c and d, Dose-response relationships for inhibition of HCN4 (c) and HCN2 (d) currents by clonidine. The solid lines are the fits to the Hill equation with the following parameters: HCN4, IC50⫽9.68 mol/L, ⫽0.83; HCN2, IC50⫽8.09 mol/L, ⫽1.04. The number of experiments for each concentration is given in parentheses. The main finding of the present study is the identification of a direct inhibitory effect of the ␣2-receptor agonist, clonidine, on cardiac pacemaker channels (HCN). To uncover this effect of clonidine, we have generated mice with targeted deletions in all 3 ␣2-adrenoceptor genes. To verify that no functional ␣2-adrenoceptors remained in ␣2ABC⫺/⫺ mice, we performed a number of experiments. Radioligand binding experiments and autoradiography confirmed the absence of ␣2-adrenoceptor protein in ␣2ABC⫺/⫺ mice. ␣2-Receptors were originally described as the adrenoceptors acting in a presynaptic feedback loop to inhibit neurotransmitter release from adrenergic nerves (for review, see Starke28). Indeed, presynaptic feedback inhibition was completely deficient in ␣2ABC⫺/⫺ mice on the basis of the following results: (1) The endogenous sympathetic neurotransmitter norepinephrine could not inhibit the electrically evoked release of [3H]norepinephrine from isolated ␣2ABC⫺/⫺ atria (Figure 2c). (2) Disruption of presynaptic feedback in sympathetic nerves resulted in elevated excretion of urinary norepinephrine. (3) As a consequence of increased sympathetic norepinephrine release, blood pressure and heart rate Knaus et al Downloaded from http://circ.ahajournals.org/ by guest on June 16, 2017 Figure 8. Clonidine shifts the voltage dependence of HCN channel activation to more hyperpolarizing voltages. Activation curves of HCN4 (a), HCN2 (b), and native If from SAN cells of ␣2ABC⫹/⫹ (c) and ␣2ABC⫺/⫺ mice (d) in the absence (black symbols) and the presence (red symbols) of clonidine. Solid lines are fits to the Boltzmann equation with the following parameters: HCN4 (n⫽8): control, V0.5⫽⫺97.0 mV, k⫽10.6 mV; at 30 mol/L clonidine, V0.5⫽⫺108 mV, k⫽8.48 mV. HCN2 (n⫽9): control, V0.5⫽⫺90.4 mV, k⫽7.08 mV; at 30 mol/L clonidine, V0.5⫽⫺98.1 mV, k⫽6.30 mV. If of ␣2ABC⫹/⫹ mice (n⫽3): control, V0.5⫽⫺84.5 mV, k⫽11.8 mV; at 10 mol/L clonidine, V0.5⫽⫺108 mV, k⫽18.0 mV. If of ␣2ABC⫺/⫺ mice (n⫽6): control, V0.5⫽⫺91.9 mV, k⫽11.3 mV; at 10 mol/L clonidine, V0.5⫽⫺104 mV, k⫽13.2 mV. The differences between V0.5 values of If of SAN cells from ␣2ABC⫹/⫹ and ␣2ABC⫺/⫺ are not statistically significant. were increased in ␣2ABC⫺/⫺ mice. (4) Chronic elevation of sympathetic tone led to the typical signs of cardiac damage, left ventricular hypertrophy and fibrosis. In addition, typical pharmacological effects of ␣2-agonists, including hypotension, sedation, and analgesia, were completely absent in ␣2ABC⫺/⫺-deficient mice. Taken together, these experiments demonstrate that ␣2ABC⫺/⫺ mice do not express any functional ␣2-adrenoceptors. Most surprisingly, clonidine elicited significant bradycardic effects in vivo and in isolated atria of ␣2ABC⫺/⫺ mice. Previously, some authors have reported that clonidine inhibited spontaneous beating frequency in isolated atria or Langendorff heart preparations.29,30 The bradycardic effect could be traced back to the sinus node, because clonidine also inhibited spontaneous beating frequency of isolated right atria from ␣2ABC⫺/⫺ mice. This effect was not mediated via Gi/ocoupled receptors, because pretreatment with pertussis toxin to inactivate this signaling pathway did not affect the bradycardia observed in isolated atria. In contrast, clonidineinduced bradycardia was eliminated in the presence of Cs⫹, which blocks If current. Electrophysiological measurements in SAN cells confirmed that clonidine inhibits If in the low micromolar range (IC50 values ⬇3 mol/L). Importantly, IC50 values in SAN cells were identical for both genotypes. Heterologously expressed HCN4 and HCN2 channels revealed an ⬇3-fold lower sensitivity to clonidine (IC50 values Clonidine Inhibits HCN Pacemaker Channels 7 ⬇9 mol/L). The slight difference from wild-type channels may be explained by the fact that in SAN cells, HCN channels are probably assembled with modulatory subunits and scaffolding proteins that are not present in HEK293 cell lines.31,32 Nevertheless, the affinities determined for clonidine are well in the range of those found for other blockers of If, such as ZD7288, cilobradine, or ivabradine, which all block If at low micromolar concentrations.2,33 As a consequence of blocking If, clonidine reduced the steepness of diastolic depolarization and hence the frequency of pacemaker potentials. The molecular identity of the clonidine binding site is not yet known. Hill coefficients for clonidine of ⬇1 suggest that there is only 1 binding site for this agent in the tetrameric HCN channel complex. Given that the pore blocker Cs⫹ induced a rightward shift of the clonidine binding curve, which is a hallmark of competitive binding, it is intriguing to speculate that the clonidine binding region is localized within or very close to the channel pore. One of the important questions is whether inhibition of HCN channels contributes to the pharmacological actions of clonidine in vivo. The present experiments with ECG telemetry in conscious, freely moving ␣2ABC⫺/⫺ mice demonstrated that clonidine doses starting at 10 g/kg elicited a significant bradycardic effect (Figure 4b). Most importantly, at all doses tested in the present study, the bradycardic effect of clonidine in ␣2ABC⫺/⫺ mice was 26% to 43% of its effect in wild-type mice. This indicates that in vivo inhibition of cardiac HCN channels by clonidine occurs in the same dose range as ␣2-receptor activation (ie, 10 to 300 g/kg) (Figure 4b). These clonidine doses are at the lower end of the spectrum of doses (30 g/kg to 50 mg/kg34 –36) that have previously been used to investigate ␣2-adrenoceptor–mediated functions in mice. The present findings in mice are consistent with a recent report demonstrating that clonidine elicited significant bradycardia but no hypotension in mice with a targeted mutation (D79N) in the ␣2A-adrenoceptor gene.34 –36 Furthermore, the clonidine doses applied in the present study are significantly lower than doses determined for specific If channel inhibitors. For example, the ED50 value of ivabradine, the only If channel inhibitor that has been introduced into therapy so far, is ⬇5 mg/kg in mice.33 However, it remains to be determined whether the observed bradycardia also contributes to the therapeutic effects of clonidine in humans. Clonidine doses used for antihypertensive therapy in humans are typically in the range of 150 to 900 g/d, but doses up to 3600 g/d have also been used in patients with essential hypertension.37 Interestingly, in tetraplegic patients with complete cervical spinal cord transsection and preganglionic sympathetic denervation, clonidine significantly lowered heart rate without affecting blood pressure.38 Inhibition of cardiac HCN channels by clonidine in humans may be of particular relevance during high-dose application of the drug, including rapid intravenous injection during hypertensive crisis or opioid detoxification.39 The present data do not lend support to the “imidazoline hypothesis” of the action of clonidine. We have not been able to obtain any results that are consistent with the HCN channel being an “imidazoline receptor.” According to the imidazoline hypothesis, I1 receptor agonists should lower blood 8 Circulation February 20, 2007 pressure, but they are not reported to be specific bradycardic agents.6,40 The present data are in line with previous reports that indicated that certain derivatives of clonidine, including N-allyl-clonidine (alinidine), act as specific bradycardic agents.41 In conclusion, clonidine can directly inhibit cardiac HCN pacemaker channels and elicit a strong bradycardic effect. This finding may be of great relevance for other neuronal effects of clonidine and other ligands with imidazoline structure, because HCN channels are ubiquitously expressed in the nervous system. Thus, clonidine-like drugs with imidazoline structure may become novel lead structures in the search for future HCN channel inhibitors. Acknowledgments Downloaded from http://circ.ahajournals.org/ by guest on June 16, 2017 The present study was supported by the Deutsche Forschungsgemeinschaft. We thank Dr Franz Hofmann (Technical University Munich, Munich, Germany) and Dr Norbert Klugbauer (University of Freiburg, Freiburg, Germany) for providing the DNA-encoding Cav3.1 and Nav1.7. Disclosures 16. 17. 18. 19. 20. 21. 22. 23. None. References 1. Biel M, Schneider A, Wahl C. Cardiac HCN channels: structure, function, and modulation. Trends Cardiovasc Med. 2002;12:206 –212. 2. Baruscotti M, Bucchi A, DiFranncesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther. 2005;107: 59 –79. 3. Schmitt H. The pharmacology of clonidine and related products. In: Gross F, ed. Antihypertensive Agents. Berlin, Germany: Springer; 1977: 299 –396. 4. Hoefke W, Kobinger W. Pharmacological effects of 2-(2,6dichlorophenylamino)-2-imidazoline hydrochloride, a new, antihypertensive substance. Arzneimittelforschung. 1966;16:1038 –1050. 5. Starke K, Endo T, Taube HD. Pre- and postsynaptic components in effect of drugs with ␣ adrenoceptor affinity. Nature. 1975;254:440 – 441. 6. Szabo B. Imidazoline antihypertensive drugs: a critical review on their mechanism of action. Pharmacol Ther. 2002;93:1–35. 7. Bylund DB, Bond RA, Clarke DE, Eikenburg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Strosberg AD, Trendelenburg UG. Adrenoceptors. In: The IUPHAR Compendium of Receptor Characterization and Classification. London, United Kingdom: IUPHAR Media; 1998:58 –74. 8. Philipp M, Hein L. Adrenergic receptor knockout mice: distinct functions of 9 receptor subtypes. Pharmacol Ther. 2004;101:65–74. 9. MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. Central hypotensive effects of the ␣2A-adrenergic receptor subtype. Science. 1996; 273:801– 803. 10. Link RE, Desai K, Hein L, Stevens ME, Chruscinski A, Bernstein D, Barsh GS, Kobilka BK. Cardiovascular regulation in mice lacking ␣2-adrenergic receptor subtypes b and c. Science. 1996;273:803– 805. 11. Hein L, Altman JD, Kobilka BK. Two functionally distinct ␣2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402: 181–184. 12. Bousquet P, Feldman J, Schwartz J. Central cardiovascular effects of ␣ adrenergic drugs: differences between catecholamines and imidazolines. J Pharmacol Exp Ther. 1984;230:232–236. 13. Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003; 65:453– 480. 14. Hofmann F, Biel M, Kaupp UB. International union of pharmacology: LI: nomenclature and structure-function relationships of cyclic nucleotideregulated channels. Pharmacol Rev. 2005;57:455– 462. 15. Shi W, Wymore R, Yu H, Wu J, Wymore RT, Pan Z, Robinson RB, Dixon JE, McKinnon D, Cohen IS. Distribution and prevalence of hy- 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. perpolarization-activated cation channel (HCN) mRNA expression in cardiac tissue. Circ Res. 1999;85:e1– e6. Stieber J, Herrmann S, Feil S, Loster J, Feil R, Biel M, Hofmann F, Ludwig A. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci U S A. 2003;100:15235–15240. Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem. 2001;268:1646 –1652. Ludwig A, Zong X, Stieber J, Hullin M, Hofmann F, Biel M. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 1999;18:2323–2329. Philipp M, Brede ME, Hadamek K, Gessler M, Lohse MJ, Hein L. Placental a2-adrenoceptors control vascular development at the interface between mother and embryo. Nat Genet. 2002;31:311–315. Bücheler M, Hadamek K, Hein L. Two ␣2-adrenergic receptor subtypes, ␣2A and ␣2C, inhibit transmitter release in the brain of gene-targeted mice. Neuroscience. 2002;109:819 – 826. Brede M, Nagy G, Philipp M, Sorensen JB, Lohse MJ, Hein L. Differential control of adrenal and sympathetic catecholamine release by ␣2-adrenoceptor subtypes. Mol Endocrinol. 2003;17:1640 –1646. Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, Lohse MJ, Hein L. Feedback inhibition of catecholamine release by two different ␣2-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106:2491–2496. Kirchhof P, Fabritz L, Fortmuller L, Matherne GP, Lankford A, Baba HA, Schmitz W, Breithardt G, Neumann J, Boknik P. Altered sinus nodal and atrioventricular nodal function in freely moving mice overexpressing the A1 adenosine receptor. Am J Physiol Heart Circ Physiol. 2003;285: H145–H153. Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998; 393:587–591. Wahl-Schott C, Baumann L, Zong X, Biel M. An arginine residue in the pore region is a key determinant of chloride dependence in cardiac pacemaker channels. J Biol Chem. 2005;280:13694 –13700. Mangoni ME, Nargeot J. Properties of the hyperpolarization-activated current If in isolated mouse sino-atrial cells. Cardiovasc Res. 2001;52: 51– 64. Proenza C, Yellen G. Distinct populations of HCN pacemaker channels produce voltage-dependent and voltage-independent currents. J Gen Physiol. 2006;127:183–190. Starke K. Presynaptic autoreceptors in the third decade: focus on ␣2-adrenoceptors. J Neurochem. 2001;78:685– 693. Light KE, Hughes MJ. Depressive action of clonidine on guinea pig and rabbit atrial pairs. Res Commun Chem Pathol Pharmacol. 1979;23: 433– 451. Rodgers RL, Tenner TE Jr, Laher IE, McNeill JH. Interaction of clonidine with chronotropic agents on isolated right atria. Can J Physiol Pharmacol. 1980;58:28 –33. Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, Pan Z, Wang H, Shi W, Robinson RB, El-Maghrabi MR, Benjamin W, Dixon J, McKinnon D, Cohen IS, Wymore R. MinK-related peptide 1: a  subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res. 2001;88:E84 –E87. Barbuti A, Gravante B, Riolfo M, Milanesi R, Terragni B, DiFrancesco D. Localization of pacemaker channels in lipid rafts regulates channel kinetics. Circ Res. 2004;94:1325–1331. Stieber J, Wieland K, Stöckl G, Ludwig A, Hofmann F. Bradycardic and proarrhythmic properties of sinus node inhibitors. Mol Pharmacol. 2006; 69:1328 –1337. Zhu QM, Lesnick JD, Jasper JR, MacLennan SJ, Dillon MP, Eglen RM, Blue DR Jr. Cardiovascular effects of rilmenidine, moxonidine and clonidine in conscious wild-type and D79N ␣2A-adrenoceptor transgenic mice. Br J Pharmacol. 1999;126:1522–1530. Tan CM, Wilson MH, MacMillan LB, Kobilka BK, Limbird LE. Heterozygous ␣2A-adrenergic receptor mice unveil unique therapeutic benefits of partial agonists. Proc Natl Acad Sci U S A. 2002;99: 12471–12476. Tank J, Jordan J, Diedrich A, Obst M, Plehm R, Luft FC, Gross V. Clonidine improves spontaneous baroreflex sensitivity in conscious mice through parasympathetic activation. Hypertension. 2004;43: 1042–1047. Knaus et al 37. Onesti G, Schwartz AB, Kim KE, Paz-Martinez V, Swartz C. Antihypertensive effect of clonidine. Circ Res. 1971;28(suppl 2):53– 69. 38. Kooner JS, Birch R, Frankel HL, Peart WS, Mathias CJ. Hemodynamic and neurohormonal effects of clonidine in patients with preganglionic and postganglionic sympathetic lesions: evidence for a central sympatholytic action. Circulation. 1991;84:75– 83. 39. Kienbaum P, Heuter T, Michel MC, Scherbaum N, Gastpar M, Peters J. Sympathetic neural activation evoked by mu-receptor blockade in Clonidine Inhibits HCN Pacemaker Channels 9 patients addicted to opioids is abolished by intravenous clonidine. Anesthesiology. 2002;96:346 –351. 40. Bousquet P, Feldman J, Tibirica E, Bricca G, Greney H, Dontenwill M, Stutzmann J, Belcourt A. Imidazoline receptors: a new concept in central regulation of the arterial blood pressure. Am J Hypertens. 1992;5:47S–50S. 41. Kobinger W, Lillie C, Pichler L. N-allyl-derivative of clonidine, a substance with specific bradycardic action at a cardiac site. Naunyn Schmiedebergs Arch Pharmacol. 1979;306:255–262. CLINICAL PERSPECTIVE Downloaded from http://circ.ahajournals.org/ by guest on June 16, 2017 Sympathetic control of heart rate plays an important role in the pathophysiology of arrhythmias, hypertension, coronary heart disease and chronic heart failure. At present, 3 pharmacological strategies are used in clinical medicine to reduce increased sympathetic tone, including ␣2-agonists, -adrenoceptor antagonists, and, most recently, hyperpolarization-activated cyclic nucleotide– gated (HCN) pacemaker channel inhibitors. Activation of presynaptic ␣2-adrenoceptors is the most widely accepted mechanism of action of the antisympathetic drug clonidine; however, other target proteins have been postulated to contribute to the in vivo actions of clonidine. To test whether clonidine elicits pharmacological effects independent of ␣2-adrenoceptors, we have generated mice with a targeted deletion of all 3 ␣2-adrenoceptor subtypes (␣2ABC⫺/⫺). ␣2ABC⫺/⫺ mice were completely unresponsive to the analgesic and hypnotic effects of clonidine; however, clonidine significantly lowered heart rate in ␣2ABC⫺/⫺ mice by up to 150 bpm. Clonidine-induced bradycardia in conscious ␣2ABC⫺/⫺ mice was 32.3% (10 g/kg) and 26.6% (100 g/kg) of the effect in wild-type mice. Clonidine inhibited the native pacemaker current (If) in isolated murine sinoatrial node pacemaker cells and the If-generating HCN2 and HCN4 channels in transfected HEK293 cells. Clonidine also inhibited HCN1 currents, although with significantly lower sensitivity. As a consequence of blocking If, clonidine reduced the slope of the diastolic depolarization and the frequency of pacemaker potentials in sinoatrial node cells from wild-type and ␣2ABC-KO mice. Direct inhibition of cardiac HCN pacemaker channels contributes to the bradycardic effects of clonidine gene-targeted mice in vivo, and thus, clonidine-like drugs represent novel structures for future subtype-selective HCN channel inhibitors. Direct Inhibition of Cardiac Hyperpolarization-Activated Cyclic Nucleotide-Gated Pacemaker Channels by Clonidine Anne Knaus, Xiangang Zong, Nadine Beetz, Roland Jahns, Martin J. Lohse, Martin Biel and Lutz Hein Downloaded from http://circ.ahajournals.org/ by guest on June 16, 2017 Circulation. published online January 29, 2007; Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2007 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/early/2007/01/29/CIRCULATIONAHA.106.667675.citation Data Supplement (unedited) at: http://circ.ahajournals.org/content/suppl/2007/01/29/CIRCULATIONAHA.106.667675.DC1 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/