* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2013 Q9 - Loreto Balbriggan
Survey
Document related concepts
Nuclear fusion–fission hybrid wikipedia , lookup
Nuclear fission wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Magnetometer wikipedia , lookup
Atomic nucleus wikipedia , lookup
Radiocarbon dating wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Radioactive decay wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Transcript
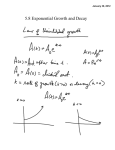
State Examination Commission – Physics Higher Level, 2013 Question 9 Define the becquerel. (6) Name one device used to detect ionising radiations. (3) Compare alpha, beta, and gamma emissions using the following headings: (a) penetrating ability, (b) deflection in a magnetic field. (9) The photograph shows one of the nuclear reactors at Chernobyl, where there was a fire in April 1986 that released large quantities of radioactive contaminants. Among the contaminants were iodine–131 and caesium–137, which are two of the unstable isotopes formed by the fission of uranium–235. Explain what happens during nuclear fission. (8) Iodine–131 decays with the emission of a beta-particle and has a half-life of 8 days. Write an equation for the beta-decay of iodine–131. Estimate the fraction of the iodine–131 that remained after 40 days. (15) Caesium–137 has a half-life of 30 years and it remains a significant contaminant in the region around Chernobyl. It is easily absorbed into the tissues of plants as they grow. Scientists collected a sample of berries growing near the abandoned power station. The activity of the sample was measured at 5000 Bq. Calculate the decay constant of caesium–137. Hence calculate the number of caesium–137 atoms present in the sample. (You may assume that all of the activity was caused by caesium–137.) (15) __________________________________________________________________________ Define the becquerel. (6) One Bq is defined as the activity of a quantity of radioactive material in which one nucleus decays per second. The Bq unit is therefore equivalent to an inverse second, s−1 Name one device used to detect ionising radiations. (3) Geiger-Muller tube Compare alpha, beta, and gamma emissions using the following headings: (a) penetrating ability, (b) deflection in a magnetic field. Penetrating power Behaviour in magnetic field α Low penetration, stopped by a few cm of air or thin sheet of paper Deflected in a way that suggests they are positively charged β Moderate penetration, most stopped by a few mm of metals like aluminium Deflected in a way that suggests they are negatively charged γ Very highly penetrating, most stopped by a thick layer of steel or concrete, but even a few cm of dense lead doesn't stop all of it. Not deflected. Explain what happens during nuclear fission. A large atomic nucleus splits into two smaller ones, comparable in size, and accompanied by the release of energy. Iodine–131 decays with the emission of a beta-particle and has a half-life of 8 days. Write an equation for the beta-decay of iodine–131. 131 131 0 53 I → 54 Xe +−1β Estimate the fraction of the iodine–131 that remained after 40 days. 40 days = 5 half lives, so the fraction remaining is 1 1 = 5 32 2 C. Garvey 2013 Caesium–137 has a half-life of 30 years and it remains a significant contaminant in the region around Chernobyl. It is easily absorbed into the tissues of plants as they grow. Scientists collected a sample of berries growing near the abandoned power station. The activity of the sample was measured at 5000 Bq. Calculate the decay constant of caesium–137. ln2 0.693 −10 −1 λ= = = 7.32×10 s t1 30×365×24×60×60 2 Hence calculate the number of caesium–137 atoms present in the sample. (You may assume that all of the activity was caused by caesium–137.) dN Rate of decay, = −λ N dt dN dt −5000 N = = = 6.83×10 12 atoms −λ −7.32×10−10 C. Garvey 2013