* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download NHS ester - BroadPharm
Survey
Document related concepts
Transcript
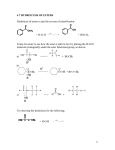
NHS ester labeling of amino biomolecules O Biomolecule NH2 + label O NHS Biomolecule N H label NHS esters and pentafluorophenyl esters are the common activated esters that are suitable for modification of amino groups which exist in proteins, peptides, amino-biomolecules, and DNA. The reaction of NHS esters with amines is pH-dependent. At low pH, the amino group is protonated, and no modification takes place. At pH that is higher than optimal, the quick hydrolysis of NHS ester can result in low yield of modification. Optimal pH value for the modification is 8.3-8.5. Water is the most common solvent for labeling. If NHS ester being used is poorly soluble in water, it can be dissolved in DMSO or DMF, and then added to a solution of protein in water. DMF must not contain amines, and thus should have no odor. The following protocol is recommended for the labeling of biomolecules with NHS esters. 1. Calculate required amount of NHS ester. 2. Determine volume of reaction mixture. 3. Dissolve NHS ester in DMSO or DMF. Amine-free DMF is preferred. 4. Dissolve biomolecule in buffer with pH 8.3-8.5. 0.1 M Sodium bicarbonate solution or 0.1 M phosphate buffer can be used. Note pH is the most important thing. Avoid using buffers containing amines (Tris can sometimes be used but not recommended). When doing large-scale labeling (hundreds of milligrams of NHS ester), note that the mixture tends to acidify with time because of hydrolysis of NHS ester. Monitor pH, or use more concentrated buffer then. 5. Add NHS ester solution to the solution of biomolecule, and vortex well. Keep on ice overnight or at room temperature during at least 4 hours. 6. Purify the conjugate using appropriate method such as gel-filtration (macromolecules is most universal). Precipitation and chromatography is another alternative. Organic impurities (such as N-hydroxysuccinimide, NHS ester, acid produced by hydrolysis) are almost always easily separated. For proteins and nucleic acids, ethanol or acetone precipitation can be used.