* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Print - Circulation
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Heart failure wikipedia , lookup
Jatene procedure wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Myocardial infarction wikipedia , lookup
Electrocardiography wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
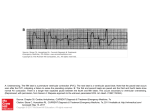
1441 Focal Mechanisms Underlying Ventricular Tachycardia During Prolonged Ischemic Cardiomyopathy Steven M. Pogwizd, MD Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 Background The present study was performed to define the mechanisms underlying spontaneously occurring ventricular arrhythmias in the failing heart. Methods and Results Three-dimensional cardiac mapping from as many as 232 intramural sites was performed in five dogs with ischemic cardiomyopathy induced by multiple intracoronary embolizations. After 5 to 10 weekly embolizations with 90-,um latex microspheres into the left anterior and circumflex coronary arteries, left ventricular ejection fraction decreased from 63 ±3% to 22+3%. Subsequent weekly Holter monitoring of dogs in the conscious state demonstrated frequent multiform premature ventricular complexes (PVCs) (.2000/d), couplets, and runs of ventricular tachycardia (VT) (<70 beats in duration and <560 beats per minute) in all dogs. Three-dimensional mapping of spontaneous rhythm for 60 minutes was performed an average of 6.4 weeks after the last embolization, during which time each dog demonstrated multiform PVCs, couplets, and runs of nonsustained VT at rates comparable to those in the conscious state. Mapping was of sufficient density to define the mechanism for 31 PVCs, 45 beats of ventricular couplets, and 99 beats of VT. Sinus beats preceding PVCs (n=36) initiated in the septum and spread rapidly with a total activation time (46±1 milliseconds) that did not differ from that of sinus beats not preceding PVCs or VT (46±2 milliseconds, n= 10, P=.69). PVCs and the first beat of couplets or VT were initiated primarily in the subendocar- dium by a focal mechanism, based on the lack of intervening electrical activity between the termination of the preceding (sinus) beat and initiation of the ectopic beat (225±23 milliseconds), despite the presence of multiple intervening intramural recording sites. All subsequent beats of VT also were initiated by a focal mechanism, and their total activation time (89±1 milliseconds) did not differ from that of the initiating ectopic beats (86±2 milliseconds, P=.14), despite acceleration of VT to a cycle length of 200 milliseconds. Monomorphic VT was due to repetitive focal activation of subendocardial sites. Polymorphic VT was due to sequential focal activation from multiple sites arising in the subendocardium and, at times, in the subepicardium. Comparison of mapping data with results of detailed anatomic analysis of tissue demonstrated that focal sites of initiation exhibited patchy nontransmural fibrosis. Functional conduction delay of a moderate degree was evident only when fibrosis extended transmurally. Conclusions Spontaneously occurring PVCs, couplets, and VT in a model of ischemic cardiomyopathy are initiated and maintained by focal mechanisms with no evidence of macroreentry. Thus, therapeutic regimens to treat or prevent VT in the presence of ischemic cardiomyopathy will likely require interruption of these focal mechanisms. (Circulation. 1994;90:1441-1458.) Key Words * cardiomyopathy * tachycardia * mapping * arrhythmias C ongestive heart failure is a lethal disorder, with a 2-year mortality approaching 60% for class III and IV patients.12 Nearly half of these deaths occur suddenly,3 primarily from ventricular tachycardia (VT) degenerating to ventricular fibrillation (VF).4 Current antiarrhythmic therapy is nonspecific, ineffective,5 and potentially proarrhythmic6,7 and can cause hemodynamic and clinical deterioration.8 Development of effective therapy will require delineation of the precise electrophysiological mechanisms involved. However, the electrophysiological mechanisms of ventricular arrhythmias in the failing heart are unknown. In fact, very little is known about the electrophysiological alterations in the failing heart because of the lack of suitable experimental preparations exhibiting stable heart failure with spontaneous arrhythmias. Hypertrophied myocardium has been studied in vitro, and the findings of heterogeneous electrical abnormalities9'10 as well as the occurrence of delayed afterdepolarizations and triggered activity" suggest, albeit indirectly, that reentrant or nonreentrant focal mechanisms, or both, may be responsible. Although left ventricular hypertrophy, a common feature of failing myocardium, is potentially arrhythmogenic,'2 the electrophysiological substrate of failing myocardium, with or without hypertrophy, is likely to be more complex. Electrophysiological alterations require definition in an experimental preparation of heart failure that demonstrates the altered substrate for arrhythmogenesis as manifested by spontaneously occurring ventricular arrhythmias. Recently, Sabbah and colleagues'3 developed a preparation of ischemic cardiomyopathy in dogs in which heart failure is induced by multiple sequential intracor- Received December 17, 1993; revision accepted May 23, 1994. From the Cardiovascular Division, Department of Internal Medicine, Washington University School of Medicine, St Louis, Mo. Reprint requests to Steven M. Pogwizd, MD, Cardiovascular Division, Box 8086, Washington University School of Medicine, 660 South Euclid Ave, St Louis, MO 63110. C) 1994 American Heart Association, Inc. onary embolizations. In addition to a marked decrease in systolic function and pathological alterations'3 that resemble ischemic cardiomyopathy in humans,14 these dogs demonstrate spontaneously occurring ventricular arrhythmias, including nonsustained VT.'1 These features make it a suitable model with which to study arrhythmogenesis in the failing heart. Elucidation of the mechanisms underlying the development of VT in this 1442 Circulation Vol 90, No 3 September 1994 I : s W! P HOLT ER i~ ii -2 4 v 4 s ' A.. K - Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 h: m -.:01::-.::1 1 1 1 1:1 FIG 1. Diagrams of intramural recording sites. Basal aspect of each transverse section for dogs 2 (left) and 4 (right) is shown. Sections through IV are oriented with base of heart on top and apex on bottom. Right ventricular (RV) and left ventricular (LV) cavities are labeled on basalmost section of map from dog 2. Black dots indicate location of LV, RV, and septal intramural recording sites (see text). preparation requires three-dimensional recording from multiple sites throughout the heart.16-20 Accordingly, the present study was performed using three-dimensional cardiac mapping in five dogs with heart failure induced by multiple intracoronary embolizations to define the mechanisms responsible for the development of spontaneously occurring ventricular arrhythmias in the failing heart. 1 1 1- * :: ::| # 1 1.1241-l 1-}.l l*X | | * l,1.1-- 1:- :: I'l--'I';l-', FIG 2. Tracings of ventricular arrhythmias during Hotter monitoring and during three-dimensional mapping of dogs 2, 4, and 5. Top, To left of each column are surface ECG tracings from Holter monitor recordings of spontaneous rhythm in conscious dogs (HOLTER); to right are lead 11 surface ECGs during open-chest three-dimensional mapping (MAPPING) of spontaneously occurring premature ventricular complexes (PVCs), couplets, and runs of ventricular tachycardia (VT) with QRS morphology and degree of complexity similar to that of ectopic activity from each respective dog. Top left, Multiform PVCs, couplets, and triplets from dog 2; top right, runs of monomorphic VT from dog 4; and middle right, PVCs and runs of monomorphic VT (with QRS morphology similar to that of PVC) and polymorphic VT from dog 5. Bottom, Surface ECG tracings from Holter monitor recordings of long runs of spontaneously occurring nonsustained VT from dogs 2, 4, and 5, respectively. Methods Embolization Protocol Animal Preparations Studies were performed on healthy male or female heartworm-negative dogs (40 to 55 lb). Ischemic cardiomyopathy was induced by a method of multiple sequential intracoronary Incidence of Spontaneous Ventricular Arrhythmias During Holter Monitoring in Conscious Dogs and During Subsequent Mapping Study Holter Maximum No. Maximum No. of Ectopic of Runs of VT Beats per Hour Dog per Hour 1 238 18 2 608 3 3 86 3 4 1849 83 5 4377 141 VT indicates ventricular tachycardia. Mapping Longest Run of VT, Beats 38 22 23 39 70 :- No. of Ectopic Beats Over 1 Hour 65 211 42 616 411 No. of Runs of VT Over 1 Hour 4 4 6 10 26 Longest Run of VT, Beats 3 3 5 7 12 ~H Pogwizd Mechanisms of Ventricular Tachycardia B A NS NS A PVC NS NS PVC n D . . .' !: _ F G Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 380 FIG 3. Three-dimensional isochronal maps from a normal sinus (NS) beat (A) and a sinus beat followed by a premature ventricular complex (PVC) (B) in dog 2. Top, Lead II surface ECGs; boxes, beats whose maps are shown below. Isochrones are drawn in 20-millisecond increments relative to initiation of each sinus beat. First 10-millisecond isochrone for each beat is indicated by dashed lines. Asterisks indicate earliest and crosses indicate latest sites of activation for each beat. embolizations.'3 There was 50% mortality usually within the first 2 weeks. Five surviving dogs underwent subsequent three-dimensional cardiac mapping. The experimental protocol was reviewed and approved by the Washington University Animal Studies Committee. A 4mvr_V[45 B 3mV - C 3 mV D I E F 3 mV 31 3mvi. G 2 my H 197 FIG 4. Initiation of premature ventricular complex (PVC) by a focal mechanism. Left, Diagram of basalmost section of PVC from Fig 3, with A through H representing selected individual intramural recording sites. Right, Bipolar electrograms from each site during a 291-millisecond interval, including termination of sinus beat to initiation of PVC. Numbers to left indicate electrode numbers and heights of autocalibrated electrogram. Vertical cursors indicate activation times listed to upper right or left, in milliseconds relative to initiation of sinus beat. After termination of sinus beat at site A, there is no electrical activity at intermediate sites B through G (or at any recording site in heart) for 275 milliseconds, until the PVC initiates at right ventricular endocardial site H, by a focal mechanism with no evidence of reentry. 1443 B C D ~~~E FIG 5. Left and right, lead 11 surface ECGs for eight multiform premature ventricular complexes (PVCs) and their preceding and subsequent sinus beats from dog 2, labeled by A through H to indicate their site of initiation. Center, Schematic of heart of dog 2, with A through H representing sites of initiation for PVCs shown. Embolization Protocol Briefly, the animals were premedicated with diazepam (0.165 mg/kg) and morphine sulfate (2.2 mg/kg), anesthetized with pentobarbital (10 mg/kg), and intubated with mechanical ventilation if needed. The lead II surface ECG was monitored on a Lifepak 4 (PhysioControl). Under sterile conditions, the distal femoral artery was isolated, and a 6F sheath was inserted for arterial access and monitoring of blood pressure (Stemtek). Left ventriculograms were performed with the animals in the left lateral position during injection of 20 mL of contrast material (lohexol, Winthrop Pharmaceuticals) as described previously20 and recorded on both 35-mm cine film and videotape. Ejection fraction was determined from the left ventricular end-systolic and end-diastolic chamber volumes, calculated by the area-length method after correction for image magnification.21 After ventriculography, the left anterior descending coronary artery (LAD) or circumflex coronary artery was selectively catheterized with a 6F Judkins catheter. A 2-mL suspension of polystyrene latex microspheres (average size, 90 ,um; range, 77 to 102 ,um) (Polysciences, Inc) was warmed to 37°C and agitated vigorously for 30 seconds; within 10 seconds, it was injected into one of the two branches of the left coronary artery along with 1 mL of contrast media. The arteriotomy was then repaired, and the skin was closed. Naloxone (0.4 mg IM) was administered, and the animal was returned to its cage. Each week, the dogs underwent a similar series of procedures with left ventriculography and catheterization with embolization of latex microspheres into the LAD or circumflex coronary artery on an alternating basis until both the inferior and anterior left ventricular walls were severely hypokinetic or akinetic. For the first 4 weeks, 2 mL of microspheres was administered, after which 3 to 6 mL was administered weekly. After the first two embolizations in dogs 1 through 3, propranolol was administered (1 mg/kg IV) in an attempt to suppress arrhythmias in the immediate postembolization period. When the ejection fraction became less than 1444 Circulation Vol 90, No 3 September 1994 NS XI X2 X3 X4 I Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 EI ¶ 86 90 67 92 408 382 275 383 CI FIG 6. Three-dimensional isochronal maps of a sinus beat (NS) and first four beats (Xl through X4) of an eight-beat run of monomorphic ventricular tachycardia from dog 4. First beat of VT, Xl, represents a fusion beat. lsochrones are drawn relative to earliest activation (*) for each beat. Listed below are total activation time (TA) and coupling interval (Cl) for each beat in milliseconds. TA 49 35% (after a mean of 7 embolizations; range, 5 to 10), no additional embolizations were performed. Ventriculograms were then performed in all dogs 4±1 weeks before their mapping study. Holter Monitoring Ambulatory Holter monitoring with animals in the conscious state was performed for 24 to 48 hours at baseline (at least 2 days before the first catheterization) and weekly beginning 2 weeks after the last embolization. The chest of each dog was shaved, ECG leads were attached, and a zippered nylon dog jacket (Alice King Chatham, Los Angeles, Calif) was applied. The Holter monitor was placed in a pocket of the jacket, and the dog's activities were unrestricted. Cassette tapes with two channels of ECG recordings were analyzed by use of a computer-assisted Marquette Series 8500 Holter analysis system to determine the frequency of premature ventricular complexes (PVCs), couplets, and runs of VT. Arrhythmia frequency was verified and edited by manual counting. Mapping Protocol Three-dimensional cardiac mapping studies were performed, as previously described,17-1922-24 an average of 6.4 weeks after the last embolization, when weekly Holter monitoring of conscious dogs consistently demonstrated frequent PVCs, occasional couplets, and runs of nonsustained VT. Briefly, dogs were anesthetized with pentobarbital (150 to 250 mg IV with additional doses of 25 to 50 mg IV as needed) and then intubated and mechanically ventilated. Body temperature was maintained at 37°C by a thermostatic esophageal probe controlling an infrared lamp. A left thoracotomy was performed, and the heart was supported in a pericardial cradle. Fifty-four plunge-needle electrodes containing four bipolar electrode pairs per needle (total of 216 intramural sites) were placed throughout the heart (Fig 1).22,23 All electrodes had an interbipole spacing of 500 gm, and the most proximal of the electrode pairs was located 500 gm from the epicardial surface. The left and right ventricular plunge-needle electrodes contained four bipolar pairs, each separated by 2.5 mm. For assessment of right ventricular activation, only the three epicardialmost pairs were consistently within the ventricular myocardium, so that the distal (endocardialmost) bipolar pairs in these regions were ignored during electrogram analysis. In dogs 4 and 5, four additional septal plunge-needle electrodes, each containing four bipolar pairs separated by 10 mm, were placed in the anterior and posterior septa under two-dimensional echocardiographic guidance. During the experiment, Pogwizd Mechanisms of Ventricular Tachycardia Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 warm (37°C) saline was applied to the heart intermittently to prevent surface cooling and to moisten the epicardium. A thermistor on the epicardial surface ensured that the epicardial temperature was maintained at 37°C. After a stabilization period of 30 minutes, bipolar electrogram information was simultaneously acquired from each of the 216 to 232 transmural sites and individually amplified, filtered (40 to 500 Hz), converted from analog to digital at a 2-kHz sampling rate, and stored, along with the lead II surface ECG tracing, on tape by use of a Sangamo Sabre IV highdensity recorder (Fairchild Weston Systems). Spontaneous rhythm was recorded continuously for 60 minutes, during which time each dog demonstrated PVCs, couplets, and runs of VT at a rate comparable to that detected in the conscious state by Holter monitoring (see "Results"). At the end of the experiment, each animal was killed by an injection of KCl. Detailed electrode localization was performed as described previously.'7-19 Each plunge-needle electrode was removed and replaced by a labeled pin. The heart was then excised and rinsed in normal saline. A polyethylene catheter (PE 60) was placed into both coronary ostia, and the arterial tree was perfused with 10% sodium phosphate-buffered Formalin. The heart was then immersed and fixed in Formalin for at least 24 hours. The pins were replaced with small plastic brush bristles to facilitate sectioning of the heart transversely into four transmural sections approximately 1.7 cm thick. Because of contraction of the heart during fixation, the cavity sizes were decreased and the wall thickness in some areas exceeded that observed during life. Each electrode was precisely localized as to its exact insertion site and the direction at which it entered the myocardium. The outline of each section was traced, showing the location of each recording site (Fig 1). Plungeneedle electrodes that lay along the plane of sectioning were represented on sections both apical and basal to the plane of sectioning. The tracings were enlarged for later three-dimensional construction of isochronal maps as described below. Spacing between plunge-needle electrodes around sites of initiation averaged 5 to 10 mm. Electrogram Analysis and Construction of Isochronal Maps Electrogram data were analyzed off-line by use of a MicroVAX-II computer (Digital Equipment Corp) with interactive color graphics. Details of the mapping analysis system have been described previously.17-192223 Initially, the tape containing the electrogram data was played back, and the accompanying surface lead II tracing was reviewed to find the beat(s) of interest (eg, sinus beat or beats of VT). Electrograms were displayed, and the activation times, assigned by the computer based on a peak criterion,1415 were reviewed and manually reassigned if required. Analyzable tracings were obtained from 97% of bipolar intramural recording sites. An amplitude threshold of 0.25 mV was considered indicative of activation of tissue by the depolarizing wave front.17-'9 After all bipolar electrograms were reviewed, the activation times for all sites were printed and assigned to their respective intramyocardial location, as indicated by the detailed three-dimensional localization described previously. Three-dimensional isochronal maps were then constructed manually in 20-millisecond increments. Serum Electrolytes and Arterial Blood Gases Peripheral venous blood samples for measurement of serum potassium (K') and magnesium (Mg2+) were obtained from conscious dogs within 1 week of the mapping study (Just before institution of ambulatory Holter monitoring) and were all within the normal range. Serum K' was measured immediately preceding administration of anesthetic (before three-dimensional mapping) and was normal. Serial arterial blood samples were obtained for blood gas and K' measurements, and any 1445 X3 LN X3 A -741 408 20 7mV B C D E F x4 | F7 2mV C l2mV V' F177 |2-20 7mV 80I 30 4mV s 1 ]3mV W1 14 d' 1 G H 3mVI~ ___ 8 lmV | Fl1-s 17 llmV I 25 Fl116 J 14mV | K 6mV | 40 L M N 4mV 1E10 44 7mV ' 111E 3mV FIG 7. Top left, Apical two sections (111 and IV) of the isochronal map of beats X3 from Fig 6 (dog 4). Top right, Schematic of sections Ill and IV from dog 4, with A through N representing individual electrode recording sites. Bottom, Bipolar electrograms from each site during a 438-millisecond interval during activation of X3 and initiation of X4. hypokalemia was corrected with intravenous KCI. Serum K' was in the normal range at the time of the mapping studies. Histological Analysis After mapping analysis, transmural tissue samples (up to 1.5 x 1.5 x 1.5 cm) from selected areas of interest from all five dogs (in particular, sites of focal initiation and regions of slow conduction) were embedded in paraffin and sectioned perpendicular to the epicardial surface at a thickness of 5 ,um. Tissue 1446 Circulation Vol 90, No 3 September 1994 NS Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 TA Xi X3 X12 53 92 84 102 328 373 396 FIG 8. Three-dimensional isochronal maps for a sinus beat (NS) and 1st (Xl), 3rd (X3), and 12th (Xl 2) beats of a 12-beat run of spontaneously occurring monomorphic ventricular tachycardia from dog 5. lsochrones are drawn relative to earliest activation (*) for each beat. Listed below are total activation time (TA) and coupling interval (Cl) in milliseconds. CI sections were stained with Masson's trichrome for examination by light microscopy. Supplies Holter monitors were generously donated by Scole Engineering (Culver City, Calif) and Delmar-Avionics (Irvine, Calif). Data and Statistical Analyses The total activation time for each beat was defined as the difference in activation time between the earliest and latest sites of activation. The mechanism responsible for a particular premature beat was defined as macroreentrant when (1) there was continuous depolarization from the preceding beat, (2) the site of initiation of a premature beat was adjacent to the site of termination of the preceding beat, and (3) the conduction velocity of the activation wave front from the site of termination of the preceding beat to the site of initiation of the ectopic beat was similar to the conduction velocity of the terminal portion of the activation wave front of the preceding beat.17-'9 A mechanism was defined as focal when the site of initiation of a premature beat demonstrated radial spread of activation and was remote from the site of termination of the preceding beat with no intervening depolarizations despite multiple (four or more) intermediate recording sites.24 A focal mechanism does not exclude the possibility of microreentry. Data are expressed as mean±SEM. Comparisons of values for ejection fraction, total activation time, and coupling interval data were performed by Student's t test (for paired or unpaired data), and P<.05 was considered indicative of a significant difference. Results Incidence of Spontaneous Arrhythmias During Ischemic Cardiomyopathy Baseline 24-hour Holter monitoring demonstrated no ventricular ectopic activity in any of the five dogs. After an average of 7 (range, 5 to 10) embolizations, the left ventricular ejection fraction decreased from 63 ±3% to 22±3% (P<.OO1). Weekly 24- to 48-hour Holter monitoring, starting 2 weeks after the last embolization, revealed a progressive increase in the degree of ventricular ectopic activity. Each dog demonstrated frequent multiform PVCs, couplets, and runs of VT (Table, left) that were either monomorphic or polymorphic (Fig 2). The maximal hourly frequency of ectopic beats and runs of VT for each dog during weekly Holter monitoring is listed in the Table (left). Dogs 4 and 5 demonstrated more frequent PVCs and runs of VT than did dogs 1 through 3 (Table); however, ejection fractions were Pogwizd Mechanisms of Ventricular Tachycardia Ill ::: 1i fli T11::!; ;; ::.; 1447 :t :: 400t CI 350 (msec) 300 250 2001 1 A Xi X2 A1 1 120 100 _ TA 1 1 Xi XI X2 X3 X4 XS X6 X7 X8 X9 XI4 1 2 80 80-. (msec) 60 40. Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 20. 2 )4 X2X3 4 'Al: 11 -JJ 400r CI (msec) 350 F )(67 ) q4)oYl X,2 FIG 9. Plots of coupling interval (Cl) and total activation time (TA) for each beat of similar QRS morphology (horizontal axis) for which every beat was mapped three dimensionally. Top, Premature ventricular complex (PVC), couplet, and runs of 3-beat and 12beat monomorphic ventricular tachycardia (VT) from dog 5. With the exception of beat X3 of the 12-beat run of VT (which is also shown in Fig 8), all of these ectopic beats were initiated at the same right ventricular subendocardial site adjacent to but distinct from the right ventricular site of initiation of normal sinus rhythm. Bottom, PVC, couplet, and runs of 3-beat and 5-beat VT from dog 4. All of these ectopic beats were initiated from a distinct right ventricular subendocardial site, distant from the posteroseptal site of initiation of normal sinus rhythm. 3001 250[ 1 200'- 1 Xi X2 120 100 TA 80 (msec) 60 40 20 0 X Xi X2 XI X2X3 X4 XS XI X2 X3 XI X2 X3 X4X5 comparable. The longest and fastest run of nonsustained VT was a 70-beat run at a rate of 560 beats per minute in dog 5 (Fig 2, bottom). Three-dimensional Mapping Three-dimensional mapping of spontaneous rhythm from as many as 232 intramural sites was performed 6.4±1 weeks after the last embolization. The technique of inserting multiple (<60) plunge-needle electrodes into the canine heart has been validated in previous in vivo studies and has been shown not to alter heart rate, mean arterial blood pressure, or cardiac output.23 In the present study, insertion of 56 to 60 plunge-needle electrodes did not alter heart rate (164±6 to 169±6 beats per minute, P=.56) or mean arterial blood pressure (101± 9 to 105±7 mm Hg, P=.30). On the surface ECG, QRS duration was unaltered. QRS morphology of sinus beats was unaltered except for a slight decrease in R-wave amplitude in two dogs, most likely due to slight anterior displacement of the heart by the posterior plunge-needle electrodes. During the 60-minute recording interval, each dog demonstrated multiform PVCs, couplets, and nonsustained runs of monomorphic or polymorphic VT with QRS morphologies (Fig 2) and frequencies (Table) that were comparable to those demonstrated on previous Holter monitoring of respective conscious dogs. However, the longest run of VT recorded during mapping was 12 beats and the fastest was a 3-beat run of VT at a rate of 268 beats per minute. PVCs and VT were more frequent during mapping in dogs 4 and 5, findings similar to those noted on Holter monitoring (Table). The three-dimensional activation sequences of 105 sinus beats, 36 PVCs, 56 beats of ventricular couplets, 124 beats of VT (from 15 runs of monomorphic VT and 16 runs of polymorphic VT), and 3 ventricular escape 1448 Circulation Vol 90, No 3 September 1994 NS PVC INIT SITE TA CI NS A 97 320 NS INIT SITE TA Cl NS Xi A NS X2 NS Xi x2 X3 I B 86 91 323 352 Xi X2 X3 NS Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 U INIT SITE TA CI A B C 97 89 88 328 362 248 A20 280 S FIG 1 0. Left, Surface ECG of a premature ventricular complex (PVC), couplet, and run of three-beat ventricular tachycardia (VT) along with preceding sinus beats (NS) from dog 2 in which there is an identical QRS morphology of the initial beats. Listed below are total activation time (TA) and coupling intervals (Cl) for each beat in milliseconds and initiation sites (INIT SITE) (A through C, right). Right, Three-dimensional isochronal maps for sinus beat (NS) and three beats (Xl through X3) of the run of three-beat VT from dog 2 (bottom left). Initiation sites of Xl through X3 (A through C, respectively) are shown in levels and 11. beats were delineated based on the analysis of more than 72 000 individual bipolar electrograms. In dogs 1 through 3, mapping was of sufficient density to fulfill criteria for arrhythmia mechanism (see "Methods") for 65% of PVCs, couplets, and VT that were analyzed. Mechanisms could not be defined for 35% of ventricular ectopic beats due to insufficient electrode density in the interventricular septum. In the two remaining dogs (4 and 5), the use of specially designed septal plungeneedle electrodes to record from 16 sites in the interventricular septum resulted in a mechanism being defined for every ectopic beat. Arrhythmia mechanisms were thus assigned for a total of 31 PVCs, 45 beats of ventricular couplets, and 99 beats of VT from the five dogs. Sinus Rhythm Sinus beats that preceded other sinus beats were initiated in the septum or the right ventricle and spread rapidly throughout the heart with no evidence of slow conduction or conduction block. The total activation time averaged 46±2 milliseconds, and the coupling interval averaged 383±+19 milliseconds (n=10). The total activation time for sinus beats from the two dogs with more frequent PVCs and VT (dogs 4 and 5, 51±3 milliseconds) was not significantly different from that from dogs 1 through 3 (43 +2 milliseconds, P=.053). An example of an activation map of a sinus beat is shown in Fig 3A. After initiation in the septum (level 111*), activation during the sinus beat proceeds rapidly to the apex and base and from endocardium to epicardium, terminating in the basolateral left ventricle in level I (+) with a total activation time of 45 milliseconds. PVCs and Couplets Sinus beats preceding PVCs exhibited activation sequences and total activation times (46±1 milliseconds, n=36) that were identical to those of sinus beats not preceding PVCs (P=.69). After termination of the sinus beats, there was no electrical activity at any recording site in the heart for 244+12 milliseconds, after which the PVCs were initiated in the subendocardium by a focal mechanism with no evidence of macroreentry. An example of the initiation of a PVC is shown in Fig 3B. The activation of the sinus beat (NS) preceding the PVC was identical to that of sinus beats shown in Fig 3A, with initiation in the septum (level 111*) and termination in the basolateral left ventricle (level It). There was no electrical activity at any recording site in the heart for 275 milliseconds, after which the PVC were initiated on the other side of the heart in the subendocardium of the right ventricle (beat PVC, level 1*) by a focal mechanism with a coupling interval of 320 milliseconds. The PVC then spread throughout the heart from the right ventricle to the left ventricle and terminated with a total activation time of 97 milliseconds. The initiation of the PVC by a focal mechanism is shown in greater detail in Fig 4. After termination of the sinus beat NS at left ventricular epicardial site A, there was no electrical activity at selected sites B to G or at Pogwizd Mechanisms of Ventricular Tachycardia Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 any other recording site in the heart for 275 milliseconds, after which the PVC was initiated at the right ventricular subendocardial site H by a focal mechanism. PVCs demonstrated only moderate conduction delay, with a total activation time averaging 83+2 milliseconds and a coupling interval of 283±9 milliseconds. Multiform PVCs arose from different subendocardial sites from the left ventricle (33% of cases), the right ventricle (53%), and the interventricular septum (14%). Although PVCs of a particular morphology usually arose from the same site, at times they could arise from one of several nonadjacent sites (Fig 5, sites E and H). Furthermore, PVCs arising at closely adjacent sites could exhibit considerably different activation patterns and QRS morphologies (sites B and C) because of marked differences in the direction of spread of their threedimensional activation wave fronts. At times, PVCs arose from the same sites at which normal sinus beats were initiated (site F) (see Fig 3A). All ventricular couplets, both monomorphic and polymorphic, were initiated and maintained by a focal mechanism arising in the subendocardium (93% of cases) or the epicardium (7%) (see "Epicardial Activation"). Sinus beats preceding couplets were identical to sinus beats preceding PVCs, with a total activation time of 46±1 milliseconds. The total activation time of the initiating beat of the couplets was 84±2 milliseconds, whereas that of subsequent beats was 87±2 milliseconds (P=.39). Initiation of very late coupled ventricular ectopic beats (isolated PVCs or the first beats of either couplets or runs of VT) occasionally led to fusion beats. The coupling interval of these fusion beats (380±11 milliseconds, n=10) was nearly identical to that of sinus beats (P=.91). The QRS morphology of the fusion beat was usually intermediate between that of sinus beats and PVCs, as was the total activation time (55±2 milliseconds compared with 46±2 milliseconds for sinus beats and 83±2 milliseconds for PVCs). VT All beats of monomorphic and polymorphic VT were initiated and maintained by a focal mechanism, which arose from the subendocardium in 96% of cases, from the midmyocardium in 1%, and from the epicardium in 3% (see below). In 6% of cases, beats of VT were initiated at the same site as earliest activation during normal sinus rhythm. Sinus beats preceding the first beats of VT were identical to sinus beats not preceding PVCs or VT, with a total activation time of 47±1 milliseconds (n=31). The total activation times of the initiating beats of VT averaged 86±2 milliseconds, whereas those of subsequent beats averaged 89±1 milliseconds (P=.14). Monomorphic VT All beats of monomorphic VT were initiated and maintained in the subendocardium by a focal mechanism. Beats of monomorphic VT arose from the left ventricle in 22% of cases, the right ventricle in 48%, and the interventricular septum in 30%. Focal activation usually arose from the same site for each beat but on rare occasions arose from a different nonadjacent site. Examples are shown in Figs 6 to 8. The isochronal maps for a sinus beat and the first four beats of an eight-beat run of VT are shown in Fig 6. The INITIATION SITE TA 54 93 104 101 99 1449 80 FIG 11. Top, Surface ECG for a sinus beat (NS) and a five-beat run of polymorphic ventricular tachycardia (Xl through X5) followed by an additional sinus beat (NS) from dog 5. Below each beat are listed its initiation site and its total activation time (TA) in milliseconds. Bottom, Schematic of heart of dog 5 with NS indicating site of initiation of sinus beats and A through C indicating sites of focal initiation of Xl through X5. sinus beat was initiated in the posterior septum in level III, with a coupling interval of 406 milliseconds, and spread rapidly with a total activation time of 49 milliseconds. The late-coupled (coupling interval, 383 milliseconds) first beat of VT, Xl, was initiated at an apical subendocardial site in level IV by a focal mechanism similar to beats X3 through X8. However, unlike X3 through X8, Xl exhibited early activation of the basal right ventricle and septum (40-millisecond isochrones in levels I to III) due to concurrent activation by a sinus beat beginning 23 milliseconds after initiation at the apex. Thus, Xl is a fusion complex with a total activa- 1450 Circulation Vol 90, No 3 September 1994 xl NS Xl I 20 i4 Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 Il t ~~~1 InI ]mX A 7 | A 7mV -4 46 . B2 B 42mVI C D 2mV 4mV 257 280 294 FIG 12. Left, Three-dimensional isochronal map of a sinus beat and first beat (X1) of a three-beat run of ventricular tachycardia that was initiated in epicardium by a focal mechanism from dog 3. Top right, Detailed view of level from isochronal map of beat Xi. Middle right, Schematic of level I from dog 3, with A indicating epicardial site of initiation of Xl and B through D indicating adjacent recording sites. Bottom right, Bipolar electrograms from A through D over a 144-millisecond interval during which Xl was initiated. tion time of 67 milliseconds, which was considerably less than that of beat X3 or X4. X2 was initiated at a distant subendocardial site in the basal posterior septum (level II) by a focal mechanism. Beats X3 through X8 were all initiated at the same apical site in level IV as Xl and exhibited nearly identical activation sequences with total activation times in the range of 86 to 92 milliseconds. The initiation of beat X3 is shown in greater detail in Fig 7. After focal initiation of X3 at site A, the depolarizing wave front spread rapidly in all directions, activating adjacent sites B through N within 44 milliseconds. Terminal activation during X3 occurred in the basal anterior right ventricle in level I (see Fig 6), 89 milliseconds after initiation at site A. There was no electrical activity at any recording site in the heart (including sites B through N) for 319 milliseconds, after which X4 was initiated at site A by a focal mechanism. Although microreentry could not be ruled out, the absence of any slow conduction or delay at or around the focal initiation site A suggests that microreentry is very unlikely. Shown in Fig 8 are the three-dimensional activation sequences for the sinus beat NS and VT beats Xl, X3, and X12 for a 12-beat run of monomorphic VT (dog 5). The sinus beat was initiated at a subendocardial site in the right ventricle (level III) and spread rapidly, terminating in the lateral left ventricle (60-millisecond isochrone, level III) with a total activation time of 53 milliseconds. There was no electrical activity at any recording site for 320 milliseconds, after which the first beat of VT, Xl, was initiated at a right ventricular site in level III, adjacent to the initiation site of the preceding sinus beat, by a focal mechanism. Beats X2 through X12 all demonstrated focal initiation at the same right ventricular site as Xl, except for beat X3, which was initiated at a posterior septal site in level II by a focal mechanism. As shown in Fig 8, X12 demonstrated not only the same initiation site as Xl but also a nearly identical activation sequence and a comparable total activation time (102 milliseconds versus 92 milliseconds for X1). Thus, monomorphic VT was initiated and maintained by repetitive focal activation, usually arising at the same subendocardial site. To elucidate possible factors influencing the development of monomorphic VT, the three-dimensional maps of PVCs, couplets, and runs of monomorphic VT that Pogwizd Mechanisms of Ventricular Tachycardia NS X, X2 X3 Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 M A B I-J, K 4mV r | L 8 n1V1 w 8 mV C M 8X 1 I D N 2 1 4-1 5 ESC FIG 13. Top, Lead II surface ECGs. Upper middle, Level II of three-dimensional isochronal maps for X3, third beat of a three-beat run of ventricular tachycardia (VT) (left), and a ventricular escape beat (ESC) that followed a three-beat run of VT (right), both of which initiated focally in the midmyocardium (*) of dog 1. Lower middle, Schematics of level 11, with A through H and K through R representing individual intramural recording sites. Bottom, Bipolar electrograms for sites A through H (left) and K through R (right) as well as from midmyocardial sites immediately basal (sites and S from level l; map not shown) and apical (sites J and T from level ll; not shown) to midmyocardial initiation site. Earliest activations arising in midmyocardium are indicated by arrow. 1I Ui 1451 I7 mv 11- 732 E F 1 G H MIS 13 P 23mV| S 27mv~| 10nm, Q _l71 R . 1.1 - 4-- 36_ . 4!!- .v T42mV a lJ Ts163 demonstrated the same QRS morphology were analyzed. Shown in Fig 9 is a summary of the total activation time and coupling interval data for such a series of beats from dog 5 (top) and dog 4 (bottom); all beats were mapped and found to be initiated by a focal mechanism arising in the subendocardium. Beats of monomorphic VT exhibited total activation times and coupling intervals that were comparable to those of isolated PVCs and couplets. In addition, late beats of VT demonstrated total activation times that were comparable to those of initiating beats, even when there was acceleration of VT as evidenced by decreasing coupling intervals (Fig 8 and Fig 9, bottom). Runs of monomorphic VT terminated after 3 to 12 beats, with the terminal beats initiating at the same subendocardial sites as preceding beats. There was no significant difference in the coupling interval between the penultimate beat (Xn -) and the terminal beat Xn (319+16 versus 286+23 milliseconds; n=15, P=.10). The total activation time of the last beat (X,) was only 9% greater than that of the penultimate beat (X, ,) (92+2 versus 84±3 milliseconds; n= 15, P=.002). Polymorphic VT Beats of polymorphic VT usually demonstrated the same morphology as isolated PVCs (Fig 2) and usually arose from the same or closely adjacent site as the PVC whose morphology it resembled. The total activation times of the initiating beats of polymorphic VT (89±+2 milliseconds, n=54) were only 7% greater than those of isolated PVCs (83±2 milliseconds, P=.01). The coupling intervals did not differ (306±15 versus 282±9 milliseconds, P=.18). To delineate how polymorphic VT developed, analysis was performed on PVCs, couplets, and runs of polymorphic VT with comparable QRS morphologies. An example is shown in Fig 10. The QRS morphologies of the PVC, the first beat of the couplet, and the first beat of the run of VT were identical, as were the second beats of the couplet and the run of VT. The activation maps for the sinus beat NS and the three beats of VT (Xl through X3) are shown in Fig 10 (right). The isolated PVC was initiated at a right ventricular subendocardial site in level I by a focal mechanism (see Fig 3B). The first beats, Xl, of the couplet and the triplet in Fig 10 (right; beat Xl, level I) was also initiated at site A, with a nearly identical coupling interval, total activation time, and activation sequence. Although no ectopic activation followed the isolated PVC, the initiating beats of the couplet and the run of VT, both labeled Xl, were followed by an ectopic beat, X2. Both 1452 Circulation Vol 90, No 3 September 1994 A X F16 C 12mV TA=48ms CI = 406 ms 29 l0-I A' 8 mV B [2XI D 426 _ 26 ~ 16 mVI A Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 A' B C D TA=91 ms Cl=343ms 28 A I3rnVI A' B 8 mV C D 12 mV 16 mV A 9 mV 2 mV| 36 17167 2 TA= 57 ms CI =383ms 9mV 50 s -/ 45 1_ 16mVW B F7-6- 2 mV 36* | fll ._28 TA= 84ms CI = 344ms C 12mV I D 16mV ` ' __ | ' ' Pogwizd Mechanisms of Ventricular Tachycardia FIG 14. Facing page. Slow conduction observed during ventricular tachycardia (VT) in dog 4. Left, Lead II surface ECGs, total activation time (TA), and coupling interval (Cl) of a sinus beat (NS) (top); first beat, Xl, of a three-beat run of VT (second panel); and first beat, Xl, of couplets (third panel and bottom) from dog 4. Center, lsochronal maps of two apicalmost sections (111 and IV) for beats enclosed in boxes on left. A, A', B, C, and D are intramural recording sites shown on isochronal map of sinus rhythm (top). Right, Bipolar electrograms over 114-millisecond intervals during activation of each beat. Activation between A and A' during first beat of VT (second panel) is markedly delayed, with more rapid continuous activation occurring from A to B to C to D to A'. Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 X2 beats were initiated focally at a basaolateral subendocardial site in level I (labeled B at left and shown at right in beat X2, level I) with no intervening electrical activity (for 265 milliseconds) at any site in the heart including the 14 intermediate sites between the termination of Xl in level 11 (t) and the initiation of X2 in level 1 (B). Both X2 beats demonstrated nearly identical total activation times, coupling intervals, and activation patterns. No ectopic activation followed X2 of the couplet. However, the second beat of VT, X2, was followed by focal initiation from a different subendocardial site in the lateral left ventricular wall (level II). X3 was activated for only 88 milliseconds, after which the tachycardia terminated. Thus, like monomorphic VT, polymorphic VT was due to consecutive focal activation, here involving multiple subendocardial sites with comparable degrees of conduction delay and prematurity. Another example of polymorphic VT is shown in Fig 11. After sequential focal activation from multiple sites (A through C) for beats Xl through X3, there was repetitive firing from one particular site (C) for beats X3 through X5, all with comparable degrees of conduction delay. This led to a monomorphic phase of the tachycardia, after which the tachycardia terminated. For all runs of polymorphic VT analyzed (n=15), the initiating and subsequent beats demonstrated similar total activation times (89±3 versus 89±2 milliseconds, P=.93) and coupling intervals (306± 15 versus 309±10 milliseconds, P=.88). Although there was no difference between the total activation times of penultimate beats (87±3 milliseconds) and those of terminal beats (87±3 milliseconds, P=.89), the coupling interval of the terminal beat was 13% less than that of the penultimate beat (287±18 versus 331±15 milliseconds, P=.04). Epicardial Activation In six cases, beats of couplets or polymorphic VT were initiated in the epicardium of the right ventricle by a focal mechanism. The total activation times averaged 99±2 milliseconds, and the coupling intervals averaged 250±23 milliseconds (n=6). An example is shown in Fig 12, with the activation map of a sinus beat and Xl, the first beat of a three-beat run of VT in dog 3. After termination of the sinus beat in the lateral left ventricle in level I (t), with a total activation time of 39 milliseconds, there was no electrical activity at any recording site in the heart for 225 milliseconds. Xl was then initiated on the other side of the heart, at a right ventricular epicardial site (site A in Fig 12B) by a focal mechanism. Activation of Xl proceeded from epicar- 1453 dium (A) to endocardium (B) and then counterclockwise (to C) and clockwise (to D). Midmyocardial Activation One ectopic beat, the third beat (X3) of a run of polymorphic VT from dog 1, was initiated from a left ventricular midmyocardial site by a focal mechanism (Fig 13, left). After termination of the preceding beat of VT, X2, at a right ventricular epicardial site in level I (not shown), there was no electrical activity at any recording site in the heart for 159 milliseconds, after which X3 was initiated at a midmyocardial site in level II by a focal mechanism. The total activation time was 104 milliseconds, and the coupling interval was 257 milliseconds. The electrograms (Fig 13, bottom left) demonstrated that midmyocardial activation at site C in level II preceded activation from both adjacent endocardial (A) and epicardial (D) sites and led to rapid spread to other adjacent sites, E through H, as well as basally to adjacent midmyocardial site I in level I and apically to midmyocardial site J in level III (maps of levels I and III are not shown). Escape Beats During mapping, three isolated ventricular escape beats occurred, each following three-beat runs of VT in dog 1. All three escape beats were initiated at the same left ventricular midmyocardial site in level II, which was different from the midmyocardial site of initiation of VT shown in Fig 13 (left). The total activation times averaged 100±1 milliseconds, and the coupling interval averaged 595 +22 milliseconds. An example is shown in Fig 13 (right). After termination of X3, the third beat of VT in level II, there was no electrical activity at any recording site in the heart for 545 milliseconds. The escape beat then initiated at a midmyocardial site L by a focal mechanism with rapid spread to adjacent endocardial (K) and epicardial sites (N). Activation then proceeded in both a clockwise and counterclockwise direction (sites 0 through R) and basally (to site S in level I, map not shown) and apically (to site T in level III, map not shown). The total activation time of the escape beat was 99 milliseconds. Conduction Slowing Although the total activation time never exceeded 104 milliseconds for any PVC or beat of couplets or VT, one dog (dog 5) occasionally demonstrated considerable local slowing of conduction in the anterior left ventricular wall during VT compared with sinus rhythm; an example is shown in Fig 14. Activation between two adjacent midmyocardial sites in level III (A and A') was very rapid (<5 milliseconds) during normal sinus rhythm (Fig 14, top). However, activation during the first beat of a three-beat run of VT (Fig 14, second panel) demonstrated conduction delay of 60 milliseconds between sites A and A', whereas activation was continuous and more rapid along the pathway A-B-C-D-A' shown at the right. Although slow conduction from A to A' was in general more frequent at shorter coupling intervals, this was not always the case. During the first beats (X1) of each of two couplets, which were initiated at a comparable coupling interval to the preceding run of VT, activation between A and A' was much more rapid (Fig 14, third panel and bottom). Thus, functional conduction delay can develop at 1454 Circulation Vol 90, No 3 September 1994 FIG 15. Low-magnification photomicrograph (left) and corresponding tracing (right) of a representative section of trichrome-stained left ventricular myocardium from cardiomyopathic dog 5. Fibrotic tissue is indicated in black. Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 short coupling intervals but appears to be dependent on other factors, such as the pattern of activation of the ectopic beat. Comparison of Pathological Specimens Pathological analysis of myocardium demonstrated areas of patchy nontransmural fibrosis throughout the distribution of the LAD and circumflex coronary artery. There was diffuse involvement of the left ventricular free wall and septum and focal involvement of the right ventricular free wall, particularly in regions close to the attachment to the septum, in all five cardiomyopathic dogs. The nontransmural fibrosis was most prominent in the subendocardium and midmyocardium but also was present in the epicardium. The pattern and extent of fibrosis are shown in a representative trichrome-stained section from the left ventricular myocardium of dog 5 (Fig 15, top). Histological sections from sites of focal initiation (n=18) were examined in detail, and distribution and extent of fibrosis were compared with those in sections from regions subtending recording sites (n=379), which were not sites of focal initiation. Sites of focal initiation were characterized by patchy nontransmural fibrosis that was most marked in the subendocardium but also involving the midmyocardium and subepicardium. The degree of fibrosis ranged from minimal to moderate but was always nontransmural and rarely involved more than 25% of transmural wall thickness. The pattern and distribution of fibrosis were characteristic not only of focal initiation sites responsible for monomorphic VT (Fig 16) or other ectopic beats but also of sites at which focal initiation did not occur (Fig 15, top). The region of slow conduction from dog 4 (shown in Fig 14) was examined. In marked contrast to areas of focal initiation, this area of slow conduction was characterized by a much greater degree of fibrosis, which was completely transmural between sites A and A' (Fig 17). Interestingly, as shown in Fig 14, conduction in this region was rapid during sinus rhythm as well as during many other ectopic beats, even at a coupling interval comparable to that at which slow conduction was ob- served. Slowing of conduction thus appeared to be dependent on both the degree of prematurity and the direction of the activation wave front, suggesting functional conduction alterations occurring in the regions surrounding the area of transmural fibrosis. Discussion Mechanism of VT The results of the present study demonstrate for the first time that spontaneously occurring PVCs and VT in a model of ischemic cardiomyopathy are initiated and maintained by a focal mechanism, without any evidence of macroreentry. There was no continuous electrical activity or intervening electrical activity between the termination of the preceding beat and the initiation of the next beat, despite the presence of multiple intervening intramural recording sites. These sinus beats preceding PVCs or VT demonstrated activation patterns and times that were identical to those of sinus beats not preceding PVCs or VT. In fact, no sinus beat ever demonstrated a total activation time of more than 58 milliseconds (based on analysis of more than 24 000 electrograms from 105 sinus beats that were mapped). Monomorphic and polymorphic VTs were due to repetitive focal initiation from either the same or different sites. Acceleration of VT was not associated with increased conduction delay or any evidence of macroreentry. The total activation time of ectopic beats never exceeded 107 milliseconds, even for runs of VT as long as 12 beats. Although microreentry within the region of focal activation cannot be excluded definitively, it is unlikely for several reasons. (1) There was no electrical activity at any intermediate intramural electrode site between the site of termination of the preceding beat and the focal initiation site (based on analysis of more than 40 000 electrograms from 175 ectopic beats), even down to a resolution of 125 gV. (2) Based on the cycle length of the tachycardias, the presence of a microreentrant circuit that was small enough to go undetected by techniques used in the present study would require a conduction velocity more than an order of magnitude slower than Pogwizd Mechanisms of Ventricular Tachycardia NS XI 1455 X2 X3 X4 X5 X6 X7 X8 X9 XIOXIt XK2 1\1,41 X x. B Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 A B FIG 16. Top, Lead II surface ECGs for the runs of monomorphic ventricular tachycardia (VT) from dogs 5 and 4, respectively (see Figs 8 and 6). In boxes are beats that were initiated at a single site by a focal mechanism. Middle, Schematics of a midapical section (level 111) from dog 5 and an apical section from dog 4 with asterisks indicating site of focal initiation of beats of VT shown in boxes above. Bottom, Trichrome-stained sections and their corresponding tracings (below) obtained from these focal initiation sites, demonstrating patchy nontransmural fibrosis similar to that observed in regions that are not focal initiation sites (Fig 15). the slowest conduction velocities measured even during VT. (3) There was no evidence of slow conduction at any recording site immediately adjacent to sites of initiation of a focus. Even with microreentrant circuits as small as those demonstrated in the infarcted canine epicardium in response to isoproterenol (as assessed by high-density grid electrode mapping),25 there was evidence of slow conduction on the order of 100 milliseconds between the initiation site and sites 1 to 2 cm away. That was not found in the present study (Fig 7). 1456 Circulation Vol 90, No 3 September 1994 .. b me a Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 FIG 17. Top left, Schematics of a region of slow conduction (a) in a midapical section (level l1l) from dog 4, along with location of a histological preparation (b) (bottom left) between midmyocardial electrode recording sites A and A' shown in Fig 14 (second panel). Right, Trichrome-stained section (b) and its corresponding tracing below, demonstrating transmural fibrosis in region between sites A and A'. For only 6% of VT beats, focal activation arose from the same site as earliest activation during sinus rhythm (terminal breakthrough sites of the left or right bundle branch). This suggests the possibility of bundle-branch reentry, which has been shown to underlie sustained monomorphic VT in about 6% of patients with dilated cardiomyopathy26 No recordings of His bundle activation were obtained during three-dimensional mapping in the present study to exclude this possibility. However, the data supporting this potential mechanism are a small percentage of the total ectopic beats analyzed. Histopathologically, focal sites of initiation demonstrated patchy nontransmural fibrosis surrounded by areas of viable myocardium, accounting for the general lack of conduction delay during sinus rhythm and VT in these hearts with severe depression of left ventricular function and spontaneously occurring VT. Although in the present study there was no slowing of overall conduction, even during long runs of VT, localized functional slowing of conduction developed in one dog (Fig 14). Here, pathological analysis demonstrated that the region of slow conduction between two electrode sites corresponded to an area of transmural fibrosis. Functional conduction delay in this area was not evident during sinus rhythm or during other ectopic beats at a comparable coupling interval in the same dog and may be due to heterogeneity of electrophysiological alterations or to alterations in anisotropic conduction27 and cellular coupling28,29 due to interstitial fibrosis in adjacent regions. Although focal mechanisms alone were found to underlie the initiation and maintenance of spontaneous VT in cardiomyopathic hearts in the present study, the presence of functional conduction delay, albeit rare, suggests that macroreentry could contribute to the development of VF, which has been shown to involve intramural reentry.17-19 Nature of Focal Mechanism The nature of the focal activation observed is unknown, but it may be due to abnormal automaticity or triggered activity arising from either delayed or early afterdepolarizations. The finding in the present study that focal activity can at times arise in the epicardium suggests a mechanism other than normal or abnormal automaticity arising from Purkinje fibers, since Purkinje fibers in the canine heart do not extend to the epicardium.3(1 Delayed afterdepolarizations have been induced in hypertrophied rat myocardium,11 the hypertensive diabetic rat heart,31 the infarcted feline heart,32 and Pogwizd Mechanisms of Ventricular Tachycardia Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 failing human myocardium in vitro.33 Myocardium from patients with end-stage heart failure demonstrates an increased duration of the calcium transient, as assessed by the bioluminescent calcium indicator aequrorin, with two distinct components reflecting impairment of calcium uptake by the sarcoplasmic reticulum.34 In addition, myocardium from failing human hearts exhibits a reduced capacity to restore low intracellular calcium levels during diastole.34 Increases in the diastolic level of calcium could turn on a transient inward current35 and lead to delayed afterdepolarizations and triggered activity. Dangman et a136 demonstrated delayed afterdepolarizations and triggered activity in the subepicardial ventricular muscle cells from the border zone of the 1-dayold infarcted canine heart. This may be related to the focal activity occasionally arising from the epicardium seen in the present study. The possibility of focal activation of an ectopic beat arising in the midmyocardium is intriguing and implicates involvement of M cells that are located in the midmyocardial region.37 These cells can demonstrate prolongation of action potential duration and, in the presence of acetylstrophanthidin or Bay K 8644, can demonstrate both delayed and early afterdepolarizations.38 The focal activation of escape beats from midmyocardial sites could also involve M cells and suggests an origin of ventricular escape activity other than from the His-Purkinje system. Canine Preparation of Heart Failure Defining arrhythmia mechanisms in the present study required an experimental preparation of heart failure that demonstrates spontaneously occurring ventricular arrhythmias, including nonsustained VT. Although a number of experimental preparations of left ventricular hypertrophy and heart failure have been developed,39 most do not demonstrate spontaneously occurring ventricular arrhythmias. The preparation of ischemic cardiomyopathy induced by multiple sequential intracoronary embolization, developed by Sabbah et al,13,15 is characterized by a progressive decrease in left ventricular systolic function, increased serum catecholamine levels, and pathological alterations comparable to those in human ischemic cardiomyopathy. In addition, this preparation is associated with marked electrophysiological alterations, including a 10% to 15% incidence of sudden death,15 and spontaneously occurring ventricular arrhythmias, including nonsustained VT.15 Whether the electrical instability demonstrated by this preparation is due to the degree of irreversible left ventricular dysfunction, the extent and/or nature of pathological alterations, or the effects of hemodynamic, neurohumoral, or other electrophysiological alterations is unknown. Nonetheless, this preparation provides a unique opportunity to determine electrophysiological mechanisms underlying arrhythmogenesis in the failing heart and could serve as a preparation for evaluation of the effectiveness of therapeutic interventions. In the present study, all five dogs that underwent cardiac mapping demonstrated, while conscious, frequent PVCs, couplets, and runs of monomorphic and polymorphic VTs (of as many as 70 beats and with rates as high as 560 beats per minute) on weekly 24- to 48-hour Holter monitoring. Although Sabbah et al'5 reported nonsustained VT in only 33% of the cardio- 1457 myopathic dogs evaluated, this was based on only one 7to 8-hour Holter monitor recording. Patients with ischemic cardiomyopathy40 exhibit considerable day-to-day variability in Holter recordings, and results have shown that one brief Holter monitor recording will fail to detect spontaneously occurring nonsustained VT in a significant number of cases.40 Three-dimensional Mapping Three-dimensional cardiac mapping of spontaneously occurring VT from as many as 232 sites and for as long as 60 minutes was possible because of the extensive data-storage capacity of the mapping system. During this interval, all of the dogs demonstrated PVCs, couplets, and occasional runs of VT with QRS morphologies and incidence that were comparable to those observed with Holter monitoring of conscious animals, not only in frequency and level of complexity but also in morphology. Mapping for 60 minutes from intramural sites throughout the left and right ventricles and the interventricular septum allowed delineation of mechanism for at least four runs of nonsustained VT for each dog and for longer runs of monomorphic and polymorphic VTs (< 12 beats) that were comparable to long runs occurring in the conscious state. Recording from the endocardial or epicardial surfaces alone would have left a large portion of the heart unmapped and would have precluded defining the underlying mechanism of arrhythmias in the failing heart. Findings in the present study suggest that focal mechanisms could contribute to the development of spontaneously occurring VT in the cardiomyopathic human heart. We have recently performed intraoperative three-dimensional cardiac mapping studies in 13 patients with ischemic cardiomyopathy who were undergoing surgical ablation for refractory VT.24 Although 5 of the 10 runs of sustained monomorphic VT induced by programmed electrical stimulation were initiated by intramural reentry, the other 5 runs of induced, sustained monomorphic VT arose by a focal mechanism with no evidence of macroreentry. Thus, focal mechanisms appear to play a role in the development of VT in human cardiomyopathy. The finding that spontaneous VT arises by a focal mechanism also suggests that the current use of antiarrhythmic agents directed primarily at altering conduction and the development of reentry may account for both their lack of efficacy and their proarrhythmic effects in patients with heart failure.6,7 Other factors that are related to development of ventricular arrhythmias in the failing heart, such as electrolyte imbalance, catecholamines, inotropic agents, and digitalis,3 may exacerbate arrhythmias arising from focal mechanisms since all of these factors can enhance delayed afterdepolarizations and lead to triggered activity in vivo as well as in vitro. Thus, approaches to the treatment of ventricular arrhythmias in the failing heart should be directed at focal mechanisms. Acknowledgments This work was supported in part by National Institutes of Health grant HL-46929. The author would like to thank Mr William Petty, Mr Lawrence Schwartz, Mr Jerome Peirick, and Dr Scott Beau for technical assistance; Mr Ross Henry, Ms Janey Wang, and Ms Carrie Kelly for assistance with data 1458 Circulation Vol 90, No 3 September 1994 analysis; Ms Ava Ysaguirre for preparation of the manuscript; Dr Jeffrey Saffitz for assistance with pathological analysis; Dr Kathryn A. Yamada for review of the manuscript; and Dr Peter B. Corr for invaluable support and advice throughout the project. References 1. Franciosa JA, Wilen M, Ziesche S, Cohn JN. Survival in men with severe chronic left ventricular failure due to either coronary heart disease or idiopathic dilated cardiomyopathy. Am J Cardiol. 1983; 51:831-836. 2. Massie BM, Conway M. Survival of patients with congestive heart failure: past, present, and future prospects. Circulation. 1987; 75(suppl IV):IV-11-IV-19. 3. Packer M. Sudden unexpected death in patients with congestive heart failure: a second frontier. Circulation. 1985;72:681-685. 4. Olshausen KV, Witt T, Pop T, Treese N, Bethge K-P, Meyer J. Sudden cardiac death while wearing a Holter monitor. Am J 5. 6. Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Courtois M, Kovacs SJ, Ludbrook PA. Physiological early diastolic intraventricular pressure gradient is lost during acute myocardial ischemia. Circulation. 1990;81:1688-1696. 21. Dodge HT, Sandler H, Baxley WA, Hawley RR. Usefulness and limitations of radiographic methods for determining left ventricular volume. Am J Cardiol. 1966;18:10-24. 22. Witkowski FX, Corr PB. An automated simultaneous transmural cardiac mapping system. Am J Physiol. 1984;247(Heart Circ Physiol 16):H661-H668. 23. Kramer JB, Saffitz JE, Witkowski FX, Corr PB. Intramural reentry as a mechanism of ventricular tachycardia during evolving canine myocardial infarction. Circ Res. 1985;56:736-754. 24. Pogwizd SM, Hoyt RH, Saffitz JE, Corr PB, Cox JL, Cain ME. Reentrant and focal mechanisms underlying ventricular tachycardia in the human heart. Circulation. 1992;86:1872-1887. 25. Zuanetti G, Hoyt RH, Corr PB. l3-Adrenergic-mediated influences 26. on microscopic conduction in epicardial regions overlying infarcted myocardium. Circ Res. 1990;67:284-302. Caceres J, Jazayeri M, McKinnie J, Avitall B, Denker ST, Tchou P, Akhtar M. Sustained bundle branch reentry as a mechanism of clinical tachycardia. Circulation. 1989;79:256-270. Spach MS, Kootsey JM, Sloan JD. Active modulation of electrical coupling between cardiac cells of the dog. Circ Res. 1982;51: 347-362. Spach MS, Miller WT III, Geselowitz DB, Barr RC, Kootsey JM, Johnson EA. The discontinuous nature of propagation in normal canine cardiac muscle. Circ Res. 1981;48:39-54. Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle: evidence for electrical uncoupling of sideto-side fiber connections with increasing age. Circ Res. 1986; 356-371. Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Cardiol. 1991;67:381-386. Arnsdorf MF, Bump TE. Management of arrhythmias in heart failure. Cardiol Clin. 1989;7:145-169. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators: Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406-412. Echt DS, Liebson PR, Mitchell B, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DW, and the CAST Investigators. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. NEnglJMed. 1991;324:781-788. Gottlieb SS, Kukin ML, Medina N, Yushak M, Packer M. Comparative hemodynamic effects of procainamide, tocainide, and encainide in severe chronic heart failure. Circulation. 1990;81: 30. 860-864. Aronson RS. Characteristics of action potentials of hypertrophied myocardium from rats with renal hypertension. Circ Res. 1980;47: 443-454. Cameron JS, Myerburg RJ, Wong SS, Gaide MS, Epstein K, Alvarez TR, Gelband H, Guse PA, Bassett AL. Electrophysiologic consequences of chronic experimentally induced left ventricular pressure overload. J Am Coll Cardiol. 1983;2:481-487. Aronson RS. Afterpotentials and triggered activity in hypertrophied myocardium from rats with renal hypertension. Circ Res. 1981;48:720-727. McLenachan JM, Henderson E, Morris KI, Dargie HJ. Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N Engl J Med. 1987;317:787-792. Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260(Heart Circ Physiol 29):H1379-H1384. Anversa P, Sonnenblick EH. Ischemic cardiomyopathy: pathophysiologic mechanisms. Progr Cardiovasc Dis. 1990;33:49-70. Sabbah HN, Goldberg AD, Schoels W, Kono T, Webb C, Brachmann J, Goldstein S. Spontaneous and inducible ventricular arrhythmias in a canine model of chronic heart failure: relation to haemodynamics and sympathoadrenergic activation. Eur Heart J. 1992;13:1562-1572. Ideker RE, Smith WM, Wolf P, Danieley ND, Bartram FR. Simultaneous multichannel cardiac mapping systems. PACE. 1987;10: 281-292. Pogwizd SM, Corr PB. Reentrant and nonreentrant mechanisms contribute to arrhythmogenesis during early myocardial ischemia: results using three-dimensional mapping. Circ Res. 1987;61: 352-371. Pogwizd SM, Corr PB. Mechanisms underlying the development of ventricular fibrillation during early myocardial ischemia. Circ Res. 1990;66:672- 695. Pogwizd SM, Corr PB. Electrophysiologic mechanisms underlying arrhythmias due to reperfusion of ischemic myocardium. Circulation. 1987;76:404-426. tricular wall. Circ Res. 1991;69:1427-1449. 31. Nordin C, Gilat E, Aronson RS. Delayed afterdepolarizations and triggered activity in ventricular muscle from rats with streptozotocininduced diabetes. Circ Res. 1985;57:28-34. 32. Kimura S, Bassett AL, Kohya T, Kozlovskis PL, Myerburg RJ. Automaticity, triggered activity, and responses to adrenergic stimulation in cat subendocardial Purkinje fibers after healing of myocardial infarction. Circulation. 1987;75:651-660. 33. Gilmour RF Jr, Heger JJ, Prystowsky EN, Zipes DP. Cellular electrophysiologic abnormalities of diseased human ventricular myocardium. Am J Cardiol. 1983;51:137-144. 34. Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61:70-76. 35. Wit AL, Rosen MR. Afterdepolarizations and triggered activity: distinction from automaticity as an arrhythmogenic mechanism. In: Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HE, eds. The Heart and Cardiovascular System: Scientific Foundations, ed 2. New York, NY: Raven Press, 1991:2113-2163. 36. Dangman KH, Dresdner KP Jr, Zaim S. Automatic and triggered impulse initiation in canine subepicardial ventricular muscle cells from border zones of 24-hour transmural infarcts: new mechanisms for malignant cardiac arrhythmias? Circulation. 1988;78: 1020-1030. 37. Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle: the M cell. Circ Res. 1991;68:1729-1741. 38. Sicouri S, Antzelevitch C. Afterdepolarizations and triggered activity develop in a select population of cells (M cells) in canine ventricular myocardium: the effects of acetylstrophanthidin and Bay K 8644. PACE. 1991;14:1714-1720. 39. Smith HJ, Nuttall A. Experimental models of heart failure. Cardiovasc Res. 1985;19:181-186. 40. Anastasiou-Nana MI, Menlove RL, Nanas JN, Mason JW. Spontaneous variability of ventricular arrhythmias in patients with chronic heart failure. Am Heart J. 1991;122:1007-1014. 27. 28. 29. Diego JM, Gintant GA, Liu D-W. Heterogeneity within the ven- Focal mechanisms underlying ventricular tachycardia during prolonged ischemic cardiomyopathy. S M Pogwizd Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 Circulation. 1994;90:1441-1458 doi: 10.1161/01.CIR.90.3.1441 Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 1994 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/90/3/1441 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/