* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Delivering more than just the matrix
Survey
Document related concepts
Transcript
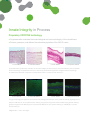
Delivering more than just the matrix ...Delivering Innate Integrity Many disease states and postoperative complications are the direct result of inflammation and the body’s consequent inability to restore a normal state of function. It is known that regenerative healing occurs in the fetal environment due to an inherent ability to modulate inflammation.8 Research has shown that this occurs because the fetal tissues, particularly the Amniotic Membrane and Umbilical Cord, have innate regenerative properties. More important, these properties can be preserved and transplanted to other environments. Our Mission . . . AMNIOX Medical is dedicated to using its proprietary technology to preserve the structural and functional integrity of fetal tissues and to deliver the benefits of this biology to patients. AMNIOX Medical is the only restorative therapy company built on the innate integrity of its advanced science, select product, sound ethics and service model. Innate Integrity in Product An innovative and unique restorative matrix • The only provider of a matrix composed of human Amniotic Membrane (AM) and Umbilical Cord (UC) • AM/UC comprises higher levels of key proteins, cytokines and growth factors that have been shown to modulate inflammation and promote regeneration of normal tissue1-6 – significantly more than Amniotic Membrane alone Biological Content: CRYOTEK Processed Human AM/UC vs. Dehydrated Human Amniochorion Matrix (dHACM)7 ® 6.3x 9.1x 50x .08x EGF KGF CRYOTEK AM/UC IL-6 HA dHACM EGF – Epidermal Growth Factor – promotes cellular differentiation, proliferation and survival KGF – Keratinocyte Growth Factor – promotes epithelialization phase of wound healing IL-6 – Interleukin-6 – strongly promotes a local inflammatory response HA – Hyaluronic Acid – regulates inflammation and aids in cell proliferation and migration Innate Integrity in Process Proprietary CRYOTEK technology • Cryopreservation maintains the innate biological and structural integrity of the natural tissue • Protects, preserves, and delivers the restorative properties of the AM/UC matrix 1. Fresh AM/UC 2. CRYOTEK AM/UC 3. Fresh HACM 4. Dehydrated HACM Cryopreservation (2) effectively maintains the structural integrity of fresh tissue (1) as demonstrated by the comparable tissue morphology. Dehydrated amniochorion sections (4) demonstrate significant changes to tissue morphology as evidenced by dramatic compaction of extracellular matrix in contrast to fresh tissue (3).7 5. Fresh AM/UC 6. CRYOTEK AM/UC 7. Fresh HACM 8. Dehydrated HACM The glycosaminoglycan Hyaluronic Acid (HA) and its proteoglycan derivative, heavy chain HA, are key signaling factors present in AM and UC. Immunofluorescence staining using HA binding protein demonstrates strong positive staining (green) throughout fresh AM/UC (5) and cryopreserved AM/UC (6), but sporadic staining in dHACM (8) in contrast to fresh tissue (7).7 *Magnification = 10x in all images. Innate Integrity in Performance • More than 25 years of NIH-sponsored research pioneering the characterization and preservation of human AM/UC • More than 130,000 transplants to date • Unparalleled pre-clinical and clinical experience documented in more than 300 peer-reviewed publications Cryopreserved Human Amniotic Membrane and Umbilical Cord CLARIX®CORD 1K Regenerative Matrix NEOX®CORD 1K Wound Matrix CR-10-1515...............................1.5 x 1.5 cm CR-10-2525...............................2.5 x 2.5 cm CR-10-4030...............................4.0 x 3.0 cm CR-10-6030...............................6.0 x 3.0 cm CR-10-8030*..............................8.0 x 3.0 cm NX-10-1515...............................1.5 x 1.5 cm NX-10-2525...............................2.5 x 2.5 cm NX-10-4030...............................4.0 x 3.0 cm NX-10-6030...............................6.0 x 3.0 cm NX-10-8030*..............................8.0 x 3.0 cm Catalog # Dimensions Catalog # Dimensions Cryopreserved Human Amniotic Membrane CLARIX®100 Regenerative Matrix NEOX®100 Wound Matrix CR-02-2020...............................2.0 x 2.0 cm NX-02-2020...............................2.0 x 2.0 cm Catalog # Dimensions CR-02-4040...............................4.0 x 4.0 cm CR-02-7070...............................7.0 x 7.0 cm Catalog # Dimensions NX-02-4040...............................4.0 x 4.0 cm NX-02-7070...............................7.0 x 7.0 cm Particulate Human Amniotic Membrane and Umbilical Cord CLARIX®FLO Regenerative Matrix Catalog # Size CR-FL-25MG.......................................25 mg CR-FL-50MG.......................................50 mg CR-FL-100MG...................................100 mg NEOX®FLO Wound Matrix Catalog # Size NX-FL-25MG.......................................25 mg NX-FL-50MG.......................................50 mg NX-FL-100MG...................................100 mg NX-FL-150MG*..................................150 mg *Available on request INNATE INTEGRITY The products offered by AMNIOX® Medical are processed and cryopreserved human Amniotic Membrane and Umbilical Cord. They are designated (and thus regulated) as a Human Cell, Tissue, and Cellular and TissueBased Product (HCT/P) by the U.S. Food and Drug Administration, are minimally manipulated and are produced in accordance with the FDA regulations for Good Tissue Practices (21 CFR 1270, 1271). The tissue procured by AMNIOX Medical is collected during live birth via Cesarean section. AMNIOX Medical procures all tissue through voluntary donation within the United States. Donor mothers are informed and consent to the procedure in advance of giving birth. The tissue is collected after the placenta has been removed from the mother’s body, so there are no risks to the patient as a result of the collection process. Our procurement process, including donor suitability, selection, and testing, meets the stringent requirements established by the Food and Drug Administration (FDA) and American Association of Tissue Banks (AATB). Donor mothers are screened at delivery for infectious, malignant, neurological and auto-immune diseases and other exposures or social habits to determine the suitability for human transplantation. Serological blood tests are performed to rule out the potential for infectious disease transmission. All tissue is processed aseptically and is tested for bacterial and fungal organisms by an independent laboratory. References 1. Liu J, et al. Expert Rev Ophthalmol (2010). 2. Chen W, et al. Wound Rep Reg (1999). 3. Koh T, et al. Expert Rev Mol Med (2011). 4. Jiang D, et al. Physiol Rev (2011). 5. Stern R. Eur J Cell (2004). 6. He H, et al. J Biol Chem (2013). 7. Data on file, Amniox Medical, Inc. 8. Namazi M, Int J Dermatol, (2011). AMNIOX Medical, CLARIX, and NEOX are registered trademarks of AMNIOX Medical, Inc. CRYOTEK is a registered trademark of TissueTech, Inc. AMNIOX Medical, Inc. – 2849 Paces Ferry Road, Atlanta, GA 30339 – 888.709.2140 – amnioxmedical.com Copyright © 2014. AMNIOX Medical, Inc. EDU-014, Rev.A