* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Tunnel Subaortic Stenosis
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Cardiac surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Aortic stenosis wikipedia , lookup
Jatene procedure wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
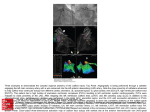
Tunnel Subaortic Stenosis Left Ventricular Outflow Tract Obstruction Produced by Fibromuscular Tubular Narrowing BARRY J. MARON, M.D., DAVID R. REDWOOD, M.D., WILLIAM C. ROBERTS, M.D., WALTER L. HENRY, M.D., ANDREW G. MORROW, M.D., AND STEPHEN E. EPSTEIN, M.D. Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 SUMMARY The clinical and morphologic features of tunnel subaortic stenosis, an unusual form of obstruction to left ventricular outflow, are described in 11 patients. Although patients with tunnel subaortic stenosis demonstrate a variety of cardiovascular malformations, the most characteristic anatomic feature is fibromuscular tubular narrowing of the outflow tract that remains relatively unchanged during the cardiac cycle. The aortic anulus was abnormally small in six of the 11 patients, including one who also had a hypoplastic ascending aorta. Evidence of a small mitral orifice was present in two patients, and two other patients had asymmetric septal hypertrophy. Although operation was successful in significantly reducing the outflow gradient in two of the seven operated patients, all seven patients had gradients of 50 mm Hg or more at the most recent postoperative evaluation. Three patients (two with previous operation) died suddenly; each of these patients had mild or no symptoms. Because of the apparent ineffectiveness of current operative methods in patients with tunnel subaortic stenosis, it is important to differentiate this condition from obstructions to left ventricular outflow. THE CLINICAL AND PATHOLOGIC FEATURES of conditions producing fixed obstruction to left ventricular outflow are well known.`8 In particular, fixed subaortic stenosis produced by a discrete fibrous membrane located just under the aortic valve has been the subject of numerous reports.'0 Subvalvular membranes of this type can be excised readily at operation, usually with relief of outflow obstruction.4' 6,8-10 However, another type of fixed subaortic stenosis has been recognized in which there is considerably more diffuse fibromuscular narrowing;2' 4, 6 8, 10-12 in our experience, this entity has very different clinical implications. In an attempt to better define this type of left ventricular outflow obstruction, which has been referred to as "tunnel aortic stenosis,"'4 we have reviewed and analyzed the clinical, pathologic, and operative findings of 11 patients with this condition. Materials and Methods Selection of Patients The operative and necropsy records of the National Heart and Lung Institute (NHLI) from 1958 to 1975 were reviewed. Eleven patients in whom the hemodynamic, angiographic, echocardiographic, operative or necropsy findings suggested the presence of diffuse obstruction to left ventricular outflow were selected for analysis. In these patients the diagnosis of tunnel subaortic stenosis was made initially by cardiac catheterization and angiography (seven patients), at operation (three patients), or at necropsy (one patient). Certain clinical findings in five of the 11 patients in this investigation have been described briefly in other reports from this institute.2' 4, 6i 1' patients (L.W. and A.G.) had only a single evaluation. At the initial cardiac evaluation (the first admission either to the NHLI or another institution) the patients ranged in age from 5 to 22 years (mean 9); at the latest cardiac evaluation or at the time of death patients ranged in age from 7 to 34 years (mean 17). The period of follow-up ranged from one to 18 years (mean 8). Seven patients were female; four were male. History and physical examination, electrocardiogram and chest radiograph were obtained at both the initial and most recent cardiac evaluations; phonocardiograms and indirect carotid pulse tracings were obtained only at the initial evaluation. Left and right heart hemodynamic studies and left ventricular angiography were performed at the initial evaluation in each of the 11 patients. In eight of the 11 patients repeat cardiac catheterization and angiographic studies were performed, including postoperative studies in seven patients. Analysis of Patients Clinical data from the initial and most recent cardiac evaluations were analyzed for nine patients. Two other From the Cardiology Branch, the Section of Pathology and the Clinic of Surgery, National Heart and Lung Institute, National Institutes of Health, Bethesda, Maryland. Address for reprints: Barry J. Maron, M.D., National Heart and Lung Institute, Cardiology Branch, Bldg. 10, Room 7B-15, Bethesda, Maryland 20014. Received April 1, 1976; revision accepted April 27, 1976. 404 Echocardiographic Studies One-dimensional echocardiographic studies were obtained at the most recent cardiac evaluation in six patients (postoperatively in three of these patients) utilizing techniques previously described;'2 two-dimensional echocardiographic studies'4 were performed in four of the six patients. In addition, one-dimensional echocardiograms were obtained in first degree relatives (the parents and three siblings) of patient R. D., who had disproportionate ventricular septal thickening in association with tunnel subaortic stenosis. Operative Procedures Ten of the 11 patients in this study underwent operation in an attempt to reduce left ventricular outflow obstruction; four of the ten patients had two operative procedures. In nine of the ten patients subaortic muscle, fibrous tissue, or both were removed at each operation. One other patient (T. Cap.) had ligation of a patent ductus arteriosus and resection of a coarctation of the aorta six years prior to attempted correction of tunnel subaortic stenosis by a left ventricular apex to descending thoracic aorta anastomosis. Two of the ten patients died at operation, K.W. at the time of her second operation and T. Cap. at the time of her apical-aortic 405 TUNNEL SUBAORTIC STENOSIS/Maron et al. anastomosis. An operation G.G. was not performed in patient Necropsy Studies Necropsy studies were performed in five patients, aged 7 23 years (average 14). Measurements were made of the maximum thicknesses of the ventricular septum, and posterior left ventricular free wall, the posterobasal left ventricular free wall (directly behind the posterior mitral leaflet), and the right ventricular wall. Blocks of myocardium were taken from the ventricular septum and left and right ventricular free walls in each heart. The tissue specimens were fixed in 10% formaldehyde, processed, embedded in paraffin, sectioned at 6g thickness and stained with hematoxylin and eosin. The severity and extent of disorganization of cardiac muscle cells was assessed by light microscopy in each tissue block."15 to Results tional class I), seven patients were in functional class II, and one patient was in functional class III (table 1). The most common symptoms were dyspnea or fatigue with exertion (six patients), chest pain that was not typical of angina pectoris (four patients), typical angina pectoris (one patient) and syncope (three patients). One patient (T. Cap.) had evidence of left and right ventricular failure and one patient (G.G.) had a history of documented paroxysmal atrial fibrillation. The age at onset of symptoms ranged from one to 29 years (median seven). Five patients have died during the period of observation. Of these, two died at operation (K.W. and T. Cap.) and three (D.A., G.G., and R.D.) died suddenly and unexpectedly. Patients D.A. and R.D. died during vigorous activity seven and 13 years after operation, respectively; patient G.G. (who did not have an operation) died during mild exertion. Patients D.A. and R.D. were asymptomatic and G.G. was in functional class II at the time of death. Physical and Phonocardiographic Findings Clinical Status Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 At the time of initial cardiac evaluation three patients asymptomatic (New York Heart Association func- were 11 Physical and auscultatory findings were similar among the patients. Each patient had a prominent, sustained left TABLE 1. Clinical and Hemodynamic Data in 11 Patients with Tunnel Subaortic Stenosis Age (yrs) Patient 5 I (Cath-1) K.W. 5 Operation-1 9 Cath-2 9 Operation-2 9 Death I (Cath-1) 5 D.A. 5 Operation-1 6 Cath-2 6 Operation-2 9 II (Cath-3) 13 Death 6 T. Cap. I (Cath-1) 7 Operation 7 Death I (Cath-1) 6 M.G. 6 Operation 9 II (Cath-2) I (Cath-1) 6 G.G. 16 Cath-2 20 Death 7 I (Cath-1) T.C. 7 Operation 9 Cath-2 II (Cath-3) 18 I (Cath-1) 9 R.D. 10 Operation 10 Cath-2 23 Death 9 I (Cath-1) L.W. 10 Operation 10 I (Cath-1) A.G. 10 Operation I (Cath-1) 16 M.S. Operation-1 16 17 Cath-2 31 Cath-3 Operation-2 31 32 Cath-4 34 II I (Cath-1) 22 A.D. 23 Operation 23 Cath-2 F FC* 1 M 3 2 Sex Age onset symptoms (yrs) CI (L/min/m2) RV S/D 9 2.8 20/1 4.1 - - - - 5.8 28/3 28/12 6.0 24/4 24/8 4 PA-RV PSG 1 19/12 PA S/D Mean LAP LVSP LVEDP LV-SA PSG Circumstances ECG (11) 220 - 120t LVH - - 190 11 95 LVH - 19 160 19 60 LVI - 240 9 130 (12) 180 9 (28) 240 30 65 140$ LBBB F 1 3 1 3.6 75/10 75/52 0 0 M 3 2 2 5.4 26/6 24/14 2 (14) 200 14 110§ M 1 1 19 - 35/4 60/4 33/11 25/12 2 35 (13) 14 240 200 20 10 140 110 60/3 30/10 30 (10) 245 32 107 LVIH RAE LVH RAE 2 F M 2 2 2 2 RBBB, LVH _ 27/3 26/9 1 5 250 18 160¶ LVH - - 24/6 2 - - (11) 10 135 185 210 10 14 10 30 60 120 LBBB LVH - - - - - 320 10 200 2 5.1 30/3 30/15 0 (15) 245 20 150 LVH LVH 7 - - 22/10 1 2 F 2 9 4.3 40/11 37/18 3 (20) 220 20 110 LVH F 1 29 - - - - 16 275 18 155 LVH - 13 165 230 23 65 93 - 155 260 18 50 16 150** F 2 2 14 1.7 2.0 24/9 - 3.6 22/7 22/12 - 0 (10) Suddenly while swimming At operation Suddenly while walking upstairs Suddenly while F - At operation LVHI 4.1 2.8 1 of death IVCD LVH LVH playing basketball 150 14 260 4.8 24 1 II *Functional class at time of death signifies patient's symptomatic status during period of time just prior to death. mm Hg subaortic pressure gradients. t55 mm Hg peak systolic pressuire gradient across aortic valve; also, 25 mm Hg and 40 mm Hg subaortic pressure gradients. 50 mm Hg peak systolic pressure gradient across aortic valve; also, 40 mm Hg and 50 §40 mm Hg peak systolic pressure gradient across aortic valve; also, 35 mm Hg and 35 mm Hg subaortic pressure gradients. ¶10 mm Hg peak systolic pressure gradient across aortic valve; also, 150 mm Hg subaortic pressure gradient. **45 mm Hg peak systolic pressure gradient across aortic valve; also, 105 mm Hg subaortic pressure gradient. I = initial cardiac evaluation; Cath-1 = first cardiac catheterization; Cath-2 = second cardiac catetherization; II = latest cardiac evaluation; ( ) pulmonary arterial wedge pressure; - = data not available. Abbreviations: CI = cardiac index; ECG = electrocardiogram; FC = functional class (New York Heart Association); IVCD = intraventricular conduction = left ventricular hypertrophy; defect; LAP = left atrial pressure; LBBB = left bundle branch block; LVEDP = left ventricular end-diastolic pressure; LVH LVS left ventricular systolic pressure; PA = pulmonary artery; PSG = peak systolic gradient; RBBB = right bundle branch block; RV = right ventricle; SA = systemic artery; S/D = systolic/diastolic pressure; RAE = right atrial enlargement. 406 CI RCULATION VOL 54, No 3, SEPTEMBER 1976 E E 0. LV BODY 1 LV OUTFLOW a 1- AORTA FIGURE 1. Pressure tracing recorded in patient (T. Cap.) with tunnel subaortic stenosis during withdrawal of an endhole catheter from the left ventricle to the aorta. Systolic pressure in the body ofthe left ventricle was 240 mm Hg. Three distinct pressure gradients were present; two within the left ventricle (40 mm Hg and 50 mm Hg) and one across the aortic valve (50 mm Hg). Simultaneously recorded electrocardiogram is shown at the top. Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 ventricular impulse and a harsh, grade 3-4/6 systolic ejection murmur that was loudest at the second to fourth left intercostal space; in nine patients a grade 1-3/6 early diastolic, decrescendo, blowing murmur was also present. The second heart sound split normally with the respiratory cycle in two patients, split paradoxically in three patients, and was single in six patients. A third heart sound was present in five patients and a fourth heart sound was present in two patients. A systolic ejection click was recorded by phonocardiography in one patient. The left ventricular ejection time (corrected for heart rate) was prolonged compared to normal standards"7 18 in seven of the eight patients studied (range 350 to 440 msec; normal 280-340 msec) and was normal in one. The left ventricular upstroke time (corrected for heart rate) ranged between 50 and 180 msec (normal 60-110 msec) and was abnormally short"9 in one patient. The contour of the peripheral arterial pulse (evaluated by either indirect carotid pulse tracing or direct intra-arterial pressure recording in the aorta or brachial artery) was, however, normal in each patient with tunnel subaortic stenosis. gradient measurements ranged from 50 Hg (median 96). The outflow gradient did not change appreciably (<25 mm Hg) in three patients, increased in two by 30 and 80 mm Hg, and decreased in two by 100 and 105 mm Hg. However, in no patient was the outflow gradient less than 50 mm Hg at the time of the latest postoperative cardiac evaluation. operative pressure to 200 mm 200r 180F 160h 14C E E z 12C _ w CE Hemodynamic Findings The hemodynamic data for the 11 patients with tunnel subaortic stenosis are summarized in table 1. At the initial (preoperative) evaluation, each patient had a marked peak systolic pressure gradient between the left ventricle and aorta (60 to 160 mm Hg; median 120). In four of these patients, a single pressure gradient was present in the subaortic area. Two other patients showed, in addition to the subaortic gradient, a second gradient across the aortic valve (10 mm Hg in patient T.C. and 45 mm Hg in patient A.D.). In each of three other patients (T. Cap., K.W. and M.G.), gradients were recorded at three levels of the left ventricular outflow tract; one gradient across the aortic valve and two separate gradients in the subaortic area (table 1, fig. 1). Left ventricular end-diastolic pressure (recorded in ten patients at the initial evaluation) was elevated (> 12 mm Hg) in eight patients and normal in two patients. Changes in left ventricular outflow gradient produced by operation in seven patients (for whom both preoperative and postoperative hemodynamic data are available) are summarized in table 1 and figure 2. The most recent post- 3r. 0 -J LL. soII_ HS8C 0 : : I-J bUf __ 40F 20C I II PREOPERATIVE POSTOPERATIVE FIGURE 2. Preoperative and postoperative left ventricular outflow gradients in seven patients with tunnel subaortic stenosis. *Patients who survived two operations. In these patients with two operations the initial preoperative measurement and the most recent postoperative measurements are given. t = died. R.D. died suddenly 13 years after operation; K. W. died at her second operation, four years after the initial operation; T. Cap. died at operation, seven years of age. TUNNEL SUBAORTIC STENOSIS/Maron et al. Angiographic Findings The angiographic appearance of the left ventricular outflow tract was similar in each patient (fig. 3). The characteristic angiographic feature (that was usually visualized best in the posteroanterior projection) was a long tubular narrowing of the left ventricular outflow tract extending proximally from the aortic anulus. This narrowing was relatively fixed (i.e., did not change appreciably in diameter during the cardiac cycle) in each patient. In six patients (M.G., K.W., D.A., A.D., A.G., and M.S.) the left ventricular cavity was greatly reduced in size during endsystole with obliteration of the apex (fig. 3B and 3D), an angiographic feature similar to that frequently observed in patients with ASH.'9 22 In each patient the aortic valve leaflets appeared thickened. Moderate aortic regurgitation was documented by aortography in two patients (A.G. and L.W.). The ascen- 407 ding aorta was normal-sized or slightly dilated in ten patients and markedly reduced in size in one (T. Cap.; figs. 3E, F). In addition, a mild coarctation of the aorta (the residua of a coarctation resection performed six years previously) was present (fig. 3E). One patient (M.S.) showed moderate mitral regurgitation. Electrocardiograms Electrocardiograms obtained at the initial evaluation were similar in each patient and showed marked left ventricular hypertrophy with T wave inversion in the left precordial leads as well as diffuse ST segment and T wave abnormalities. At the latest evaluation, electrocardiograms were essentially unchanged in six patients; however, three other patients developed conduction defects following operation (left bundle branch block in two and intraventricular conduction defect in the other). Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 FIGURE 3. Left ventricular angiocardiograms demonstrating the anatomic abnormalities in tunnel subaortic stenosis. A, B and C from patient M.G. D from patient A.G.; E and F from patient T Cap. A) Angiocardiogram in diastole (posteroanterior view) showing long tubular narrowing of the left ventricular outflow tract (arrowv) and markedly thickened left ventricular free wall. B) A ngiocardiogram in end-systole (posteroanterior view) showing long tubular narrowing of the left ventricular outflow tract that is relatively unchanged from that seen in diastole (compare with 3A) and obliteration of the left ventricular apex. C) Angiocardiogram in systole (lateral view) showing narrowing of the left ventricular outflow tract (arrows). D) Angiocardiogram in systole (posteroanterior view) showing long narrowing of left ventricular outflow tract that appears eccentrically located and obliteration of the apex. E) Angiocardiogram in diastole (posteroanterior view) showing dilated, irregularly shaped left ventricular cavity, narrowed outflow tract, hypoplasia of the ascending aorta, mild coarctation of the aorta (arrow) and dilatation of the coronary arteries. F) Angiocardiogram in systole (posteroanterior view) showing narrowing of the left ventricular outflow tract that is unchangedfrom that seen in diastole (compare with 3E). 408 CIRCULATION Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 Uit ; VOL 54, No 3, SEPTEMBER 1976 0tii00000t2 FIGcURE 4. Echocardiographic tracing shown at three levels of the heart, representing selections from a continuous scan from mitral valve to aorta. This record is from patient T C. with tunnel subaortic stenosis, II years after operation, an outflow gradient of 60 mm Hg was demonstrated shortly after the echo was obtained. A) at the level of the tips of the mitral leaflets- B) cephalad to the mitral valve in the subaortic area (i.e., the left ventricular outflow tract), C) at the level of the aortic valve. In A the distance between the ventricular septum and mitral valve at onset of systole is normal and the mitral valve appears to be normally positioned in the left ventricular cavity. The ventricular septal thickness is only slightly increased over normal; this may be due to the fact that the echocardiogram was taken aJter operation (during which large amo unts of m uscle were resected from the left ventricular outftlow tract). A t the level sho wn in C, the ao rtic anulus is normal-sized. At the level shown in B, however, the left ventricular outflow tract is markedly narrowed. Ao V =aortic valve leaflet; A WOT an terior wall of left ventricular outflow tract; (i.e., the cephalad portion of the ventricular septum); A WA = anterior wall of aorta; EKG electrocardiogram; LA = left atrial cavity, L VOT - left ventricular outflow tract; MV = mitral valve; PW = posterior left ventricular free wall; PWA posterior wall of aorta; PWOT = posterior wall of left ventricula r outflow tract (i.e., the cephalad p ortio n of the mitral valve), R V right vientricular cavity; RVO right ventricular outflow tract; VS ventricular septum. Calibration mark equals 10 mm. = = = = Chest Radiographs At initial cardiac evaluation, the heart size as determined by chest radiograph was normal (cardiothoracic ratio 0.55) in seven patients and enlarged in four patients. Pulmonary venous markings were increased in two patients. At the most recent evaluation, the heart size had increased in one patient (M.S.) and decreased in another (G.G.). = Echocardiographic Findings In the three patients studied preoperatively (T. Cap., A.G. and L.W.) and the one patient who did not undergo operation (G.G.), one or two-dimensional echocardiograms showed the left ventricular outflow tract to be markedly narrowed. The aortic anulus* was abnormally small" in T. *We have used the term aortic anulus to refer to that portion of the aorta at the level of the cephalad extension of the commissures, This area of the aorta is also known as the sinotubular junction. Cap. and L.W. and normal-sized in G.G. and A.G. In addition, two other patients (A.D. and T.C.) were studied by one and two-dimensional echocardiography eight and 11 years after operation, respectively. Both of these patients also showed a markedly narrowed left ventricular outflow tract (fig. 4); the aortic anulus was abnormally small in A.D. and normal-sized in T.C. In five of the six patients studied echocardiographically the ventricular walls were concentrically thickened. One patient (G.G.) had echocardiographically documented asymmetric septal hypertrophy (ASH) (ventricular septal thickness of 28 mm, posterobasal left ventricular wall thickness of 20 mm and septal-free wall ratio of 1.4). In addition, the mitral leaflets were anteriorly displaced in the left ventricular cavity at the onset of systole (fig. 5), a finding previously reported in patients with obstructive ASH24 as well as in patients with other conditions."' 26 Abnormal systolic anterior motion of the mitral valve occurred in TUNNEL SUBAORTIC STENOSIS/Maron et 409 al. leaflet showed a reduced E-F slope. In addition, the presence of an abnormally decreased and relatively fixed distance between the anterior and posterior mitral leaflets during suggested diastole the presence of a reduced mitral valve orifice in two patients (T. Cap. and A.D.). Although cardiac catheterization data in both these patients suggested that hemodynamically significant mitral stenosis was not present, direct operative examination of the mitral valve in T. Cap. revealed the mitral orifice to be abnormally small. The transverse left atrial dimension was normal23 in three of the six studied. The left atrium patients mm) in one patient (T. Cap.) was decreased in size (20 and increased in patient G.G. (49 mm) and patient A.G. (35 mm). The echocardiographic assessment echocardiograms Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 FIGURE 5. Echocardiogram from patient G. G. with tunnel subaortic stenosis showing disproportionate thickening of the ventricular septum (VS) with respect to the posterobasal left ventricular wall (PW). The mitral valve is displaced forward in the ventricular cavity at the onset of systole. Calibration mark equals 10 mm. patient R.D. was con- not have had normal-sized left atria at necropsy. One-dimensional of Cap. and 6.6. patients who did of left atrial size in T. firmed at necropsy. Three other echocardiogram obtained in the father (who had disproportionate ventricular septal thickening in addition to tunnel subaortic stenosis) showed asymmetric septal hypertrophy with a septal thickness of 23 mm, posterobasal left ventricular wall thickness of 12 mm and septal-free wall ratio of 1.9. Echocardiograms performed on the mother and two siblings of patient R.D. were normal. Studies on the family members of patient G.G. could not be performed. Operative Findings and Procedures patient G.G. (fig. 5), as well as in patient A.G. who did not have disproportionate septal thickening. In each of the six patients with echocardiographic studies, the anterior mitral The findings at operation and the operative procedures performed in ten of the 11 patients with tunnel subaortic stenosis are summarized in table 2. In each of nine patients TABLE 2. Operative Data in Ten Patients with Tunnel Subaortic Stenosis Patient Thickened aortic valve leaflets Age (yrs) K.W.t D.A.t 9 6 T. Cap§ 7 Operative findings Thickened mitral Small aortic valve anulus + + 0 0 leaflets + + Operative Procedures Diffuse narrowing of LVOT* + + - - Resection of fibrous tissue from LVOT Resection of ventricular muscle from LVOT Aortotomy + + + + (small amount) + 0 0 0 Other Left ventriculotomy 0 Anastomosis between LV apex and descending thoracic aorta utilizing a dacron prosthesis incorporating a porcine xenograft M.G. 6 + 0 T.C. R.D. 7 10 0 + + M.S.¶ A.G. 31 23 10 + 0 + L.W. 10 + A.D. + + + + + - 0 + 0 0 + + + + + + + + + + + (small amount) + + + 0 0 0 + 0 + Excised large amt. LV myotomyof thickened ectomy as performed in endocardium ASH27 overlying LV + 0 Left ventriculotomy Incision inito fibrous tissue + + + + thicik fibrous and muscular tissue forming a ring around LVOT, involving the anterior mitral leaflet and extendling about 2 cm proximal to tA previous operation was performed at another institution at age 5 years; subaortic fibrous tissue was resected and left ventrictular myotomy performed. fib)rous tissue was resected. tA previous operation was performed at another i nstitution a t age1 5 years; a small amouint of subaortic §A previous operation wvas performed at another institution at age year; a patent Iuictus arteriosus wias ligatedl and coarctation of aorta repaired. ¶A previous operation 'was performed at NIHLI at age 16 years; subaortic fibrous tissue was resected alnd an aortic valvotomy performed. - = Not described. + = Performed or present. 0 = Not performed or not present. Abbreviations: LV left ventricular; LVOT = left ventricular outflow tract; VS ventricular septum; amt amount. *A long area of the aortic anulus. = = = 410 CI RCU LATION VOL 54, No 3, SEPTEMBER 1976 TABLE 3. Necropsy Findings in Five Patients with Tunnel Subaortic Stenosis Age Heart wt (NL limit)28 Max VS Max LVFW Wall thickness (mm) VS-LVPB ratio LVPB* Pulmonic (NL range)29, 4.0 (4.1-6.5) 6.5 Patient (yrs) Sex K.W. (A67-14) D.A. (A72-115) T. Cap. (A74-125) 10 F 325g 17 17 17 1.0 4 13 M (200) 690g (300) 23 23 23 1.0 6 7 F 240g (150) 16 16 16 1.0 10 G.G. 20 M 830g (350) 35 35 30 1.2t 10 720g 30 (A73-109) R.D. M 23 (5.8X6.6) 5.0 (3.0-6.2) 7.5 (7.0-9.0) 20 20 1.5 (350) (A74-331) Valve circuLmference (cm) Aortic U (NL range)29, s Max RV 6 3.2 (3.6 6.0) 4.5 (4.8 6.4) 2.3 (3.2-5.6) 6.0 Size LA NL NL 1 (6.0-7.5) 7.0 4.3 (7.0-9.0) (6.0-7.5) NL modG rading system: 0 = normal cellular arrangement or absence of myocar(dial scarring; 1+ mild cellular disorganization or mild myocardial scarring; 2+ erate cellular disorganization or moderate myocardial scarring; 3+ = severe cellular disorganization or severe myocardial scarring. *Measured behind the posterior leaflet of the mitral valve. [Although the septal-free wnall ratio obtained at necrospy in this patient (i.e., 1.2) 'was not indicative of disproportionate septal thickening, tle septal-free wnall ratio obtained by echocardiography (in diastole) wnas 1.4. In patients wnithi disproportionate septal thickening, the posterobasal left ventricuilar xvall thickens considerably more than the ventricular septum in systole and postmortem hearts are often fixed in the systolic phase of the cardiac cycle. Suich considerations probably explain the disparity between the septal-free wall ratios obtained in this patient (luring life as compared to those obtained at necropsy. = - decreased. - increased; left ventricular papillary muscles; left ventricular outflow tract; LV pap left ventricular; LVFW - left ventricular free wall; LVOT Abbreviations: LV w ventricular septal; W t LVPB - left ventricular posterobasal wall; max maximum; NL normal; RV rig ht ventricular; SE su bendocardiial; VS weight. = = = Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 undergoing an operation in which the left ventricular outflow tract was visualized and subaortic tissue was removed, diffuse narrowing of the outflow tract extending for an estimated 2 cm proximal to the aortic valve was present. The ventricular muscle in the outflow tract was greatly hypertrophied and was covered by a thick layer of fibrous tissue that usually extended over the anterior mitral leaflet. In five patients (K.W., D.A., M.G., A.G. and T.C.) both fibrous tissue and underlying ventricular muscle was resected from the left ventricular outflow tract. In three patients (M.S., L.W., and A.D.) only fibrous tissue was removed and in one patient (R.D.) an incision was made into the fibrous tissue of the outflow tract but no tissue was removed. The left ventricular outflow gradient increased after operation in R.D. Necropsy Findings The necropsy findings in five hearts with tunnel subaortic stenosis are summarized in table 3 and illustrated by representative examples in figs. 6-10. Heart weights uniformly were markedly increased compared to normal standards.28 The gross anatomic features of the left ventricular outflow tract were similar in each of the five hearts. The out- = flow tract was markedly narrowed in an area extending about I to 3 cm proximal to the aortic anulus (fig. 6B, 7C, 8, 9A-B, and 10). This relatively long area of narrowing was associated with marked thickening of the ventricular muscle (figs. 6B, 7C, 8, and 9A). The muscle in the left ventricular outflow tract was covered by a thick layer of fibrous tissue that invariably extended onto the anterior leaflet of the mitral valve (figs. 9B and 10). In four patients (K.W., D.A., T. Cap., and R.D.) the aortic anulus was abnormally small (figs. 6A, B, 7C, 8, 9A-B and 10) compared to normal standards;28" 2 the aortic valve leaflets showed fibrous thickening in each patient (figs. 6A, 9B, and 10). In one patient (T. Cap.) the ascending aorta was markedly reduced in size (fig. 7A). In addition, patient G.G. (who had a 35 mm Hg systolic pressure gradient between the right ventricle and the pulmonary artery) showed hypertrophy of the crista supraventricularis. The left ventricular walls were markedly increased in thickness in each patient. In four of the five patients the thickening was concentric, i.e., the ventricular septal to posterobasal left ventricular wall thickness ratio was < 1.3 (figs. 6B and 7C). In one patient (R.D.) disproportionate FIGURE 6. Heart of patient K.W. (A67-14). A) Bicuspid aortic valve viewed from above showing considerable fibrous thickening of the leaflets. B) Longitudinal cut showing the left atrium (LA), thickened anterior (A) and posterior (P) mitral leaflets, left ventricular (L V) free wall, right ventricular (RV) wall, ventricular septum (IVS) and aorta. The left ventricular outflow tract and cavity are markedly narrowed and the ventricular septal thickness is equal to that of the left ventricular free wall. TUNNEL SUBAORTIC STENOSIS/Maron et al. Thickened aortic valve leaflets TSmall aortic anulus Thickn mitral valve leaflets Narrowed Narred scarred 411 Disorganization Ventricular scarring of cardiac LVOT VS LVFXV LVpap RV Vs muscle cells LVFW EV + + + + 2+ 2+ 3+ 0 0 0 0 + + + + 1+ 1+ 1+ 0 0 0 0 + + + + 3+ 1+ 2+ 3+ 0 0 0 +t 0 + + 2+ 2+ 3+ 0 3+ 0 0 + + + + 3+ 3+ 2+ 0 1+ 0 0 Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 thickening'3 of the ventricular septum was present (with a ventricular septal to posterobasal left ventricular wall thickness ratio of 1.5) (fig. 8). Histologic examination of myocardium from the ventricular septum and ventricular free walls showed virtually all cardiac muscle cells to be hypertrophied and normally arranged with respect to each other in three of the five patients. G.G. showed disorganization of hypertrophied cardiac muscle cells in the ventricular septum; disorganization of cardiac muscle cells was not present in the left ventricular free wall of this patient. R.D., who had disproportionate ventricular septal thickening, showed only minimal disorganization of cardiac muscle cells in the ventricular septum. Scarring of ventricular myocardium (including both replacement and interstitial fibrosis) was present in all five patients but was particularly marked in two (T. Cap. and R. D.; fig. 7C). The scarring usually involved the ventricular septum and left ventricular free wall, always included the papillary muscles, and was usually located in the subendocardial region. Abnormalities of the intramural coronary arteries30 were present in each patient. These abnormalities were focal and usually consisted of both intimal proliferation and medial hypertrophy; some involved arteries had narrowed lumens. Abnormal intramural coronary arteries were present in areas of scarring as well as in areas without scarring, were generally more numerous in the ventricular septum than in the left ventricular free wall, and were absent or rare in the right ventricle or atria. The extramural coronary arteries in each patient had wide-open lumens. The anatomic abnormalities in patients with tunnel subaortic stenosis (as determined by angiographic, echocardiographic, operative and necropsy observations) are summarized in table 4. FIGURE 7. Heart of patient T. Cap. (A 74-125). A) Exterior view showing the ascending aorta to be hypoplastic compared to the pulmonary trunk (PT). R. A. right atrium. B) Exterior view of = heart showing the dacron prosthesis that was inserted into the apex of the left ventricle (L V) at operation. RV right ventricle. C) Longitudinal section of heart showing small left and right ventricular cavities, narrow left ventricular outflow tract, ventricular septal (VS) thickness that is equal to that oJ the left ventricular (L V)free wall and markedly thickened right ventricular wall. Note that in this figure (as in figs. 8-10) the left ventricular free wall appears to curve toward, and is in a position close to the cephalad aspect of the ventricular septum. This relation of ventricular septum to left ventricular free wall was observed in several patients with tunnel subaortic stenosis when the heart was sectioned through the anterolateral papillary muscle but not when the heart was sectioned between the papillary muscles as shown diagrammatically in figs. 11 and 12. There is marked fibro us scarring of the ventricular septum and right ventricular free wall (R V). Scarring in the left ventricle is limited to the papillary muscles. Both mitral and aortic valves are also thickened by fibrous tissue. Area of left ventricular apex used for anastomosis is shown by dotted line. D) View from above the left atrium (LA) and right atrium (RA). The left atrium is small and the right atrium and coronary sinus are dilated. The walls of both atria are thickened. CI RCULATION 412 VOL 54, No 3, SEPTEMBER 1976 Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 sent diverse manifestations of a single disease, we cannot exclude the possibility that tunnel subaortic stenosis is a morphologic abnormality common to several etiologically distinct diseases. Nonetheless, the characteristic tubular narrowing of the outflow tract present in our patients clearly distinguishes tunnel subaortic stenosis from aortic valvular stenosis and supravalvular aortic stenosis. Discrete (membranous) subaortic stenosis, the form of left ventricular outflow obstruction most likely to be confused with tunnel subaortic stenosis, is characterized by a relatively thin (1-2 mm in thickness), crescent-shaped fibrous membrane that extends across the anterior portion of the left ventricular outflow tract (with each end inserting onto the anterior mitral leaflet) about I cm below the aortic anulus. It should be pointed out, however, that it may be possible for discrete (membranous) subaortic stenosis and tunnel subaortic stenosis to occur in the same patient. We have observed several patients (not included in this study) with a typical discrete subaortic fibrous membrane, who also had evidence of diffuse narrowing of the left ventricular outflow tract. Indeed, we believe it is likely that patients with discrete subaortic stenosis may demonstrate a spectrum of abnormalities of the left ventricular outflow tract ranging from mild deformity to the severe narrowing of typical tunnel subaortic stenosis. The importance of clinically differentiating a patient with tunnel subaortic stenosis from one with a discrete subaortic membrane relates to the efficacy of operation in reducing the gradient in these two conditions. In patients with a discrete subaortic membrane (with or without severe obstruction of the left ventricular outflow tract) marked operative relief of outflow obstruction usually can be accomplished with low risk.4'' I In contrast, of the patients reported in this paper with tunnel subaortic stenosis, the smallest residual gradient present at the most recent cardiac evaluation was 50 mm Hg. In five of seven operated patients (in whom serial measurements of the outflow gradient were made) the magnitude of subaortic obstruction increased or did not change appreciably following operation; outflow obstruction decreased significantly after operation in only two patients. There was no obvious factor that might explain why operation reduced the outflow gradient in some patients with tunnel subaortic stenosis and not in others. However, it may be relevant that four of the five patients with poor operative results had an abnormally small aortic anulus, while the anulus was not small in either of the two patients with relatively favorable operative results. The poor prognosis of patients with tunnel subaortic stenosis is emphasized by the fact that three patients died suddenly and unexpectedly (two FIGURE 8. Heart ofpatient R.D. (A 74-331). Longitudinalsection showing that the ventricular septum (VS) is disproportionately thicker than the left ventricular (L V) free wall. The left ventricular outflow tract is narrowed but the left ventricular cavity appears relatively normal in size. A V aortic valve; Ao. = aorta, LAA left atrial appendage; RV= right ventricle. = e, Discussion Each of the patients with tunnel subaortic stenosis described in this study showed, by definition, severe tubular narrowing of the left ventricular outflow tract which was morphologically similar from patient to patient. In addition, the aortic anulus was abnormally small in over one-half of our patients, a finding which in one patient was associated with hypoplasia of the ascending aorta and coarctation of the aorta. Two patients had asymmetric septal hypertrophy. Systolic anterior motion of the mitral valve was present in one of these patients, as well as in another patient who did not show asymmetric septal hypertrophy. Two other patients had evidence of a small mitral orifice. Although this broad spectrum of abnormalities may repre- TABLE, 4. Anatomic Abnormalities* in 11 Patients with Tunnel Subaortic Stenosis Tubular LV outflow tract Abnormally small aortic anulus Hypoplastic ascending aorta Small mitral orifice Asymmetric septal hypertrophy SAM K.W. D.A. + + + + 0 0 0 0 0 0 - T. Cap. + + + + 0 0 M.Q. + 0 0 0 - G.G. Patients T.C. R.D. L.W. M.S. A.D. A.G. + + + 0 0 0 + 0 0 + ± ± Ot + + 0 + 0 0 0 0 0 0 0 0 0 + + 0 + 0 0 - 0 *Based on the synthesis of angiographic, echocardiographic, operative and pathologic data. was judged to be slightly small at operation but appeared normal-sized on an echocardiogram recorded 11 years later. + - Abnormality bresent. O -Abnormality absent. - - No data available. Abbreviations: LV = left ventricular; SAM - abnormal systolic anterior motion of the anterior mitral leaflet (demonstrated by echo). tAortic anulus 0 + 0 0 0 ± 413 TUNNEL SUBAORTIC STENOSIS/Maron et al. K Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 FIGURE 9. Heart ofpatient R.D. (A74-331). A) Close-up view of left ventricular outflow tract. A thick fibrous plaque is present in apposition to the anterior mitral leaflet. B) View with the aorta opened showing the aortic valve (A V) and subvalvular area. The anterior mitral leaflet (A ML), in apposition to the mural endocardial plaque, also is thickened. The aortic valve cusps are also diffusely thickened by fibrous tissue. The area of the aortic valve anulus, as well as the outflow tract, appear abnormally narrowed. LAA left atrial appendage; L V left ventricle. of whom died late postoperatively). Another form of left ventricular outflow obstruction that may be confused with tunnel subaortic stenosis is obstructive ASH. Patients with obstructive ASH characteristically demonstrate disproportionate thickening of the ventricular septum. Also, the mitral valve is positioned anteriorly in the left ventricular cavity and obstruction to outflow occurs because of an abnormal systolic anterior motion of the tip of the anterior mitral leaflet. While these features may also occur in some patients with tunnel subaortic stenosis (figs. 5 and 8), the left ventricular outflow tract (that area just below the aortic valve) is not markedly deformed in patients with FiGURE 10. Heart ofpatient D.A. (A 72-115). With aorta opened, showing aortic valve and subvalvular area. Both the outflow tract and the area of the aortic anulus are greatly reduced in size. In addition, both mitral and aortic valve cusps are thickened by fibrous tissue that also covers the mural endocardium in the left ventricular outflow tract. The chordae tendineae attached to the anterior mitral leaflet are also thickened. A V aortic valve; A anterior mitral leaflet, LYV left ventricle. 414 CIRCULATION NORMA L Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 DISCRETE SUBAORTIC STEN OSIS ASYMMETRIC SEPTAL HYPERTROPHY - OBSTRUCTIVE (IHSS) VOL 54, No 3, SEPTEMBER 1976 FIGURE 11. Diagrammatic illustration of the salient anatomic findings of patients with various types of subaortic obstruction, including discrete (membranous) subaortic stenosis, obstructive ASH [typical idiopathic hypertrophic subaortic stenosis (IHSS)I and tunnel subaortic stenosis. Although the anatomic variation in tunnel subaortic stenosis is too great to permit summation in a single illustration, the heart at the bottom shows the most common features of this condition - i.e., a markedly narrowed left ventricular outflow tract, concentrically thickened ventricular walls, an abnormally small aortic anulus, normal-sized mitral orifice and left atrium and a mitral valve positioned normally in the left ventricular cavity. Each heart is shown sectioned between the papillary muscles and in the mid-point of the posterior mitral leaflet; the hearts are depicted during the mid-diastolic phase of the cardiac cycle. Anterolateral papillary muscles are shown by dotted lines for purposes of orientation. Fibrous tissue is shown in solid black. Ao = aorta; AML - anterior mitral leaflet; LA = left atrium; LV left ventricle; LVOT= left ventricular outflow tract; PM = papillary muscle (anterolateral); PML = posterior mitral leaflet; PW= posterior left ventricular wall; VS = ventricular septum. TUNNEL SUBAORTIC STENOSIS ASH, as can be appreciated on angiographic study or at necropsy. Although most patients with genetically transmitted ASH can be easily distinguished clinically from patients with tunnel subaortic stenosis, our findings suggest that on occasion these disease entities may be related. For example, patient R.D. with tunnel subaortic stenosis had disproportionate ventricular septal thickening at necropsy, and ASH was documented echocardiographically in his father. Patient G.G. had both unequivocal ASH on echocardiographic study and severe disorganization of cardiac muscle cells in the ventricular septum, a histologic finding that is characteristic of genetically transmitted ASH."5 16 Thus, two of our 11 patients with tunnel subaortic stenosis had findings suggestive of genetically transmitted ASH,',15, 116, 20, 31-34 raising the possibility that fibromuscular narrowing of the left ventricular outflow tract may represent another manifestation of the disease spectrum of ASH. The anatomic features of hearts with discrete subaortic stenosis and obstructive ASH are compared to a representative heart with tunnel subaortic stenosis in diagrammatic form in figure 11. However, the broad spectrum of anatomic abnormalities present in patients with tunnel subaortic stenosis cannot be satisfactorily summarized in a single diagram. Therefore, in figure 12 three variations of anatomic abnormalities seen in patients with tunnel subaortic stenosis (including the one shown in fig. 11) are illustrated during both the diastolic and systolic phases of the cardiac cycle. This description is not intended to represent a strict classification of abnormalities in tunnel subaortic stenosis, but rather emphasizes the anatomic variability in patients with this condition. By definition, all patients with tunnel subaortic stenosis (i.e., diagrams A, B, and C in fig. 12) manifest diffuse narrowing of the left ventricular outflow tract. Typically, these patients also have an abnormally small aortic anulus, but normal-sized mitral orifice and left atrium; concentric ventricular wall thickening is usually present with a mitral valve that is normally positioned in the left ventricular cavity (diagram B). In contrast, in some patients (diagram A, representing patient T. Cap.) the aortic anulus, mitral orifice and left atrium are each abnormally small (in addition to the narrowed outflow tract). Finally, patients may demonstrate disproportionate ventricular septal thickening, abnormal systolic anterior motion of the mitral valve, and a mitral valve positioned anteriorly in the left ventricular cavity. These patients also have an enlarged left atrium, but normal-sized aortic anulus (diagram C, representing patient G.G.). The clinical features of our patients are similar to those of the 12 patients described by Kelly et al.8 as having a form of TUNNEL SUBAORTIC STENOSIS/Maron et al. A. 415 g B. . FIGURE 12. Diagrammatic illustrations showing the spectrum of anatomic abnormalities present in patients with tunnel subaortic stenosis. Each heart is shown sectioned between the papillary muscles and in the mid-point of the posterior mitral leaflet; the hearts are depicted during the mid-diastolic and mid-systolic phases of the cardiac cycle. Anterolateral papillary muscles are shown by dotted lines for purposes of orientation. Appearance of the heart in the midsystolic phase corresponds generally to that observed at necropsy. Fibrous tissue is shown in solid black. Ao = aorta; AML = anterior mitral leaflet; LA = left atrium; LV= left ventricle; L VOT = left ventricular outflow tract; PM = papillary muscle (anterolateral), PML = posterior mitral leaflet; PW = posterior left ventricular wall; VS = ventricular septum. B. .......j ....~~~~ Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 ....... M I D - DIASTOLE X.... MID- SYSTOLE discrete subaortic stenosis (Type II in his report). However, while these investigators used the term tunnel aortic stenosis to describe only those patients with both fibromuscular narrowing of the outflow tract and an abnormally small aortic anulus, we prefer to regard all patients with fibromuscular narrowing of the outflow tract (regardless of the size of the aortic anulus) as part of the disease spectrum of tunnel subaortic stenosis. Deutsch et al.7 also described the angiographic features of one patient who had a "tunnel-like stricture" of the left ventricular outflow tract. Two other patients have been described35 who demonstrated fibromuscular narrowing of both the right and left ventricular outflow tracts at necropsy. In addition, fibromuscular tunnel deformity of the left ventricular outflow tract in patients with complete transposition of the great vessels has been described.36 current operative methods in patients with tunnel subaortic stenosis, it is important to differentiate this condition from other forms of obstruction to left ventricular outflow. References 1. 2. 3. 4. 5. 37 In conclusion, this report describes the clinical, hemodynamic and morphologic features of an unusual form of obstruction to left ventricular outflow, tunnel subaortic stenosis. Patients with tunnel subaortic stenosis demonstrate a variety of congenital cardiovascular malformations, although the most characteristic finding is tubular left ventricular outflow tract narrowing that remains relatively unchanged during the cardiac cycle. This fibromuscular deformity of the outflow tract often is associated with a small aortic anulus. Because of the apparent ineffectiveness of 6. 7. 8. 9. 10. 11. Glancy DL, Epstein SE: Differential diagnosis of type and severity of obstruction to left ventricular outflow. Prog Cardiovasc Dis 14: 153, 1971 Morrow AG, Roberts WC, Ross J Jr, Fisher RD, Behrendt DM, Mason DT, Braunwald E: Obstruction to left ventricular outflow. Current concepts of management and operative treatment. Ann Intern Med 69: 1255, 1968 Edwards JE: Pathology of left ventricular outflow tract obstruction. Circulation 31: 586, 1965 Reis RL, Peterson LM, Mason DT, Simon AL, Morrow AG: Congenital fixed subvalvular aortic stenosis. An anatomical classification and correlations with operative results. Circulation 43, 44 (suppl I): I-ll, 1971 Roberts WC: Valvular, subvalvular and supravalvular aortic stenosis: morphologic features. Cardiovasc Clin 5: 104, 1973 Fisher RD, Mason DT, Morrow AG: Results of operative treatment in congenital aortic stenosis. Pre- and postoperative hemodynamic evaluations. J Thorac Cardiovasc Surg 59: 218, 1970 Deutsch V, Shem-Tov A, Yahini JH, Neufeld NH: Subaortic stenosis (discrete form). Radiology 101: 275, 1971 Kelly DT, Wulfsberg E, Rowe RD: Discrete subaortic stenosis. Circulation 46: 309, 1972 Spencer FC, Neill CA, Sank L, Bahnson HT: Anatomical variations in 46 patients with congenital aortic stenosis. Am Surgeon 26: 204, 1960 Morrow AG, Goldblatt A, Braunwald E: Congenital aortic stenosis. II. Surgical treatment and the results of operation. Circulation 27: 450, 1963 Popp RL, Silverman JF, French JW, Stinson EB, Harrison DC: Echocardiographic findings in discrete subvalvular aortic stenosis. Circulation 49: 226, 1974 416 CIRCULATION Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 12. Hancock EW: Differentiation of valvar, subvalvar and supravalvar aortic stenosis. Guy's Hosp Rep 110: 1, 1961 13. Henry WL, Clark CE, Epstein SE: Asymmetric septal hypertrophy: Echocardiographic identification of the pathognomonic anatomic abnormality of IHSS. Circulation 47: 225, 1973 14. Griffith JM, Henry WL: A sector scanner for real-time, two-dimensional echocardiography. Circulation 49: 1147, 1974 15. Ferrans VJ, Morrow AG, Roberts WC: Myocardial ultrastructure in idiopathic hypertrophic subaortic stenosis. A study of operatively excised left ventricular outflow tract muscle in 14 patients. Circulation 45: 769, 1972. 16. Maron BJ, Ferrans VJ, Henry WL, Clark CE, Redwood DR, Roberts WC, Morrow AG, Epstein SE: Differences in distribution of myocardial abnormalities in patients with obstructive and nonobstructive asymmetric septal hypertrophy (ASH): Light and electron microscopic findings. Circulation 50: 436, 1974 17. Benchimol A, Dimond EG, Shan Y: Ejection time in aortic stenosis and mitral stenosis. Comparison between the direct and indirect arterial tracings with special reference to pre- and postoperative findings. Am J Cardiol 6: 728, 1960 18. Golde D, Burstin L: Systolic phases of the cardiac cycle in children. Circulation 42: 1029, 1970 19. Braunwald E, Lambrew CT, Rockoff SD, Ross J, Morrow AG: Idiopathic hypertrophic subaortic stenosis. I. A description of the disease based upon an analysis of 64 patients. Circulation 29 (suppl IV): IV-1, 1964 20. Epstein SE, Henry WL, Clark CE, Roberts WC, Maron BJ, Ferrans VJ, Redwood DR, Morrow AG: Asymmetric septal hypertrophy. Ann Intern Med 81: 650, 1974 21. Simon AL, Ross J Jr, Gault JH: Angiographic anatomy of the left ventricle and mitral valve in idiopathic hypertrophic subaortic stenosis. Circulation 36: 852, 1967 22. Adelman AG, McLoughlin MJ, Marquis Y, Auger P, Wigle ED: Left ventricular cineangiographic observations in muscular subaortic stenosis. Am J Cardiol 24: 689, 1969 23. Feigenbaum H: Echocardiography. Philadelphia, Lea and Febiger, 1973, pp 216, 217 24. Henry WL, Clark CE, Griffith JM, Epstein SE: Mechanism of left ventricular outflow obstruction in patients with obstructive asymmetric sep- 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. VOL 54, No 3, SEPTEMBER 1976 tal hypertrophy (idiopathic hypertrophic subaortic stenosis). Am J Cardiol 35: 337, 1975 Nanda NC, Gramiak R, Manning JA, Lipehik EO: Echocardiographic features of subpulmonic obstruction in dextro-transposition of the great vessels. Circulation 51: 515, 1975 Bloom KR, Meyer RA, Bove KE, Kaplan S: The association of fixed and dynamic left ventricular outflow obstruction. Am Heart J 89: 586, 1975 Morrow AG, Reitz BA, Epstein SE, Henry WL, Conkle DM, Itscoitz SB, Redwood DR: Operative treatment in hypertrophic subaortic stenosis. Techniques and the results of pre- and post-operative assessments in 83 patients. Circulation 52: 88, 1975 Gould SE: Pathology of the Heart and Blood Vessels, ed 2. Springfield, Ill., Charles C Thomas, 1960, pp 1060-1064 Schultz DM, Giordano DA: Hearts of infants and children. Arch Pathol 74: 464, 1962 McReynolds RA, Roberts WC: The intramural coronary arteries in hypertrophic cardiomyopathy. (abstr) Am J Cardiol 35: 154, 1975 Menges H, Brandenburg 0, Brown AL: The clinical, hemodynamic and pathologic diagnosis of muscular subvalvular aortic stenosis. Circulation 24: 1126, 1961 Abbasi AS, MacAlpin RN, Eber LM, Pearce ML: Left ventricular hypertrophy diagnosed by echocardiography. N Engl J Med 289: 118, 1973 Teare D: Asymmetrical hypertrophy of the heart in young adults. Br Heart J 20: 1, 1958 Clark CE, Henry WL, Epstein SE: Familial prevalence and genetic transmission of idiopathic hypertrophic subaortic stenosis. N Engl J Med 289: 709, 1973 Sissman NJ, Neill CA, Spencer FC, Taussig HB: Congenital aortic stenosis. Circulation 19: 458, 1958 Shaher RM, Puddu GC, Khoury G, Moes CAF, Mustard WT: Complete transposition of the great vessels with anatomic obstruction of the outflow tract of the left ventricle. Surgical implications of anatomic findings. Am J Cardiol 19: 658, 1967 Shaher RM, Moes CAF, Khoury G: Radiologic and angiocardiographic findings in complete transposition of the great vessels with left ventricular outflow tract obstruction. Radiology 88: 1092, 1967 Sunderman FW, Boerner F: Normal Values in Clinical Medicine. Philadelphia, WB Saunders Co., 1949, p 641 Tunnel subaortic stenosis: left ventricular outflow tract obstruction produced by fibromuscular tubular narrowing. B J Maron, D R Redwood, W C Roberts, W L Henry, A G Morrow and S E Epstein Downloaded from http://circ.ahajournals.org/ by guest on June 15, 2017 Circulation. 1976;54:404-416 doi: 10.1161/01.CIR.54.3.404 Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 1976 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/54/3/404 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/