* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Heart Failure:
Herpes simplex research wikipedia , lookup
Public health genomics wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Henipavirus wikipedia , lookup
Viral phylodynamics wikipedia , lookup
Swine influenza wikipedia , lookup
Influenza A virus subtype H5N1 wikipedia , lookup
Human mortality from H5N1 wikipedia , lookup
Avian influenza wikipedia , lookup
HEALTHLINE
October 2006
Title: Focus on Pandemic Influenza
PATIENT CARE
Pandemic Influenza
A pandemic is a global disease outbreak. A flu pandemic occurs when a new influenza virus emerges for
which people have little or no immunity, and for which there is no vaccine. The disease spreads easily
person-to-person, causes serious illness, and can sweep across the country and around the world in very
short time. Unlike the annual influenza season (December – March), pandemic influenza could occur at
any time.
Flu Terms Defined
Seasonal (or common) flu is a respiratory illness that can be transmitted person to person. Most people
have some immunity, and a vaccine is available.
Avian (or bird) flu is caused by influenza viruses that occur naturally among wild birds. The H5N1 variant
is deadly to domestic fowl and can be transmitted from birds to humans. There is no human immunity and
no vaccine is available.
Pandemic flu is virulent human flu that causes a global outbreak, or pandemic, of serious illness. Because
there is little natural immunity, the disease can spread easily from person to person. Currently, there is no
pandemic flu.
When is a Pandemic likely to Occur?
It is difficult to predict when the next influenza pandemic will occur or how severe it will be. Wherever and
whenever a pandemic starts, everyone around the world is at risk. Countries might, through measures
such as border closures and travel restrictions, delay arrival of the virus, but cannot stop it. Since 1900
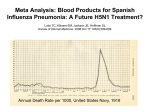
there were 3 influenza pandemics: Spanish, Asian, and Hong Kong flu in 1918, 1957, and 1968,
respectively.
Timeline of Human Flu Pandemics
Major pandemic
The appearance of a new influenza strain in the human population
Copyright 2006
All Rights Reserved
Published by Omnicare, Inc.
distributed by PBM Plus, Inc.
Page - 1
HEALTHLINE
October 2006
Health professionals are concerned that the continued spread of a highly pathogenic avian H5N1 virus
across eastern Asia and other countries represents a significant threat to human health. The H5N1 virus
has raised concerns about a potential human pandemic because:
It is especially virulent
It is being spread by migratory birds
It can be transmitted from birds to mammals and in some limited circumstances to
humans, and
Like other influenza viruses, it continues to evolve.
Since 2003, a growing number of human H5N1 cases have been reported in Azerbaijan, Cambodia,
China, Djibouti, Egypt, Indonesia, Iraq, Thailand, Turkey, and Vietnam. More than half of the people
infected with the H5N1 virus have died. Most of these cases are all believed to have been caused by
exposure to infected poultry. There has been no sustained human-to-human transmission of the disease,
but the concern is that H5N1 will evolve into a virus capable of human-to-human transmission.
Avian (bird) flu is caused by influenza A viruses that occur naturally among birds. "Human influenza virus"
usually refers to those subtypes that spread widely among humans. There are only three known A
subtypes of influenza viruses (H1N1, H1N2, and H3N2) currently circulating among humans. It is likely
that some genetic parts of current human influenza A viruses originally came from birds. Influenza A
viruses are constantly changing, and other strains might adapt over time to infect and spread among
humans.
Symptoms of avian influenza in humans have ranged from typical human influenza-like symptoms (e.g.,
fever, cough, sore throat, and muscle aches) to eye infections, pneumonia, severe respiratory diseases
(such as acute respiratory distress), and other severe and life-threatening complications. The symptoms
of avian influenza may depend on which virus caused the infection.
Because these viruses do not commonly infect humans, there is little or no immune protection against
them in the human population. If H5N1 virus were to gain the capacity to spread easily from person to
person, a pandemic (worldwide outbreak of disease) could begin. No one can predict when a pandemic
might occur. However, experts from around the world are watching the H5N1 situation very closely and
are preparing for the possibility that the virus may begin to spread more easily and widely from person to
person.
Copyright 2006
All Rights Reserved
Published by Omnicare, Inc.
distributed by PBM Plus, Inc.
Page - 2
HEALTHLINE
October 2006
For more information about human infection, see http://www.cdc.gov/flu/avian/gen-info/avian-fluhumans.htm
Vaccines and Treatments for Avian Influenza
There currently is no commercially available vaccine to protect humans against H5N1 virus that is being
seen in Asia, Europe, and Africa. A pandemic vaccine cannot be produced until a new pandemic
influenza virus emerges and is identified. The U.S. Department of Health and Human Services (HHS) is
working on development of vaccines based on existing H5N1 viruses and expanding the national capacity
for vaccine development.
The H5N1 virus that has caused human illness and death in Asia is resistant to amantadine and
rimantadine, two antiviral medications commonly used for influenza. Two other antiviral medications,
oseltamavir and zanamavir, would probably work to treat influenza caused by H5N1 virus, but additional
studies still need to be done to demonstrate their effectiveness. Therefore, as with other types of
influenza, the primary intervention is supportive treatment with fluids, and products to relieve respiratory
symptoms, cough and congestion.
What would be the Impact of a Pandemic?
A pandemic may come and go in waves, each of which can last for six to eight weeks. An especially
severe influenza pandemic could lead to high levels of illness, death, social disruption, and economic
loss. Everyday life would be disrupted because so many people in so many places become seriously ill at
the same time. Impacts can range from school and business closings to the interruption of basic services
such as public transportation and food delivery.
Seasonal Flu
Pandemic Flu
Occurs annually, usually in winter, in temperate
climates
Occurs rarely (three times in 20th century - last in
1968)
Usually some immunity built up from previous
exposure
No previous exposure; little or no pre-existing
immunity
The very young, the elderly and those with certain
underlying health conditions at increased risk for
Healthy people may be at increased risk for
serious complications
Copyright 2006
All Rights Reserved
Published by Omnicare, Inc.
distributed by PBM Plus, Inc.
Page - 3
HEALTHLINE
October 2006
serious complications
Health systems can usually meet public and patient
needs
Health systems may be overwhelmed
Vaccine developed based on known flu strains and
available for annual flu season
Vaccine probably would not be available in the
early stages of a pandemic
Adequate supplies of antivirals are usually available
Effective antivirals may be in limited supply
Average U.S. deaths approximately 36,000/yr
Number of deaths could be quite high
Symptoms: fever, cough, runny nose, muscle pain.
Symptoms may be more severe and
complications more frequent
Generally causes modest impact on society
May cause major impact on society
Manageable impact on domestic and world economy
Potential for severe impact on domestic and
world economy
What should we all be doing to prepare for Pandemic Influenza?
Health care providers will play a crucial role in the event of a pandemic. Planning for pandemic influenza
is key. The Centers for Disease Control has prepared checklists, a toolkit, and guidelines to assist health
care providers and service organizations in planning for a pandemic outbreak.
The checklist for long-term care facilities outlines a structure for planning and decision making. Based on
differences among facilities (e.g., patient/resident characteristics, facility size, scope of services, hospital
affiliation), each facility will need to adapt the checklist to meet its unique needs and circumstances. The
checklist should be used as one tool in developing a comprehensive pandemic influenza plan.
Comprehensive pandemic influenza planning can also help facilities plan for other emergency situations.
The complete checklist and educational resources can be found at www.pandemicflu.gov.
Copyright 2006
All Rights Reserved
Published by Omnicare, Inc.
distributed by PBM Plus, Inc.
Page - 4
HEALTHLINE
October 2006
The next HealthLines Newsletter will outline the critical steps Omnicare is taking to be prepared to
support residents and facilities as well as employees in the event of an influenza pandemic.
For additional information on seasonal flu visit: http://www.hhs.gov/flu/. BZ
NEW DRUGS/INDICATIONS
Exubera (insulin human [rDNA origin]) Inhalation Powder: Exubera is indicated for the treatment of
hyperglycemic control in adult patients with diabetes mellitus. The onset of action is similar to rapid-acting
insulin analogs and the duration of glucose-lowering activity is comparable to subcutaneously
administered regular human insulin. In patients with Type 1 diabetes, Exubera should be used in
Copyright 2006
All Rights Reserved
Published by Omnicare, Inc.
distributed by PBM Plus, Inc.
Page - 5
HEALTHLINE
October 2006
regimens that include a longer-acting insulin. In patients with Type 2 diabetes, Exubera can be used as
monotherapy or in combination with oral agents or longer-acting insulins.
Exubera consists of blisters containing 1 mg or 3 mg of human insulin inhalation powder, which are
administered using the Exubera Inhaler, delivering insulin by oral inhalation. The insulin is absorbed as
quickly as subcutaneously administered rapid-acting insulin analogs and more quickly than
subcutaneously administered regular human insulin in healthy subjects and in patients with Type 1 or
Type 2 diabetes. Unlike the absorption of subcutaneous regular human insulin, the absorption of insulin
following inhalation of Exubera is independent of body mass index and there are no apparent differences
in the pharmacokinetic properties of Exubera when comparing patients over the age of 65 years and
younger adult patients. Exubera differs from regular human insulin by its rapid onset of action. When used
as mealtime insulin, the dose of Exubera should be given within 10 minutes of starting a meal.
Exubera is contraindicated in patients hypersensitive to it or one of its components, in patients who
smoke or who have discontinued smoking less than 6 months prior to starting it and in patients with
unstable or poorly controlled lung disease. Note that if a patient starts or resumes smoking, Exubera must
be discontinued immediately due to the increased risk of hypoglycemia.
Because of the effect it has on pulmonary function, all patients should have pulmonary function assessed
prior to initiating therapy, after the first six months of therapy and annually thereafter, even in the absence
of pulmonary symptoms. The use of Exubera in patients with underlying lung disease, such as asthma or
COPD, is not recommended because the safety and efficacy of it in this population have not been
established. Bronchospasm has been reported rarely but patients experiencing such a reaction should
discontinue Exubera and seek medical evaluation immediately. In controlled clinical studies, 1.2% of
patients discontinued treatment due to cough and small number of patients (0.4%) discontinued treatment
due to dyspnea. A small number of patients discontinued treatment due to pharyngitis (0.2%) and
increased sputum (0.1%) and no patients discontinued treatment due to epistaxis.
Exubera Blisters are to be stored at controlled room temperature, 25ºC (77ºF). Once the foil over wrap is
opened, unit dose blisters should be protected from moisture and should be used within 3 months
(additional care should be taken to avoid humid environments, e.g. steamy bathroom following a shower).
Discard the blister if frozen. Exubera Inhalers should be cleaned per manufacturer’s guidelines once a
week for best functioning, should be stored at controlled room temperature, 25ºC (77ºF) and should not
be frozen or refrigerated. The Exubera® Inhaler can be used for up to 1 year from the date of first use.
DP
WARNINGS AND ADVERSE EFFECTS
Appropriate use of erythropoietic products is an important aspect of Omnicare’s Chronic Kidney Disease
(CKD) Health Management Program. A number of patients serviced by Omnicare have CKD associated
anemia that require costly therapies utilizing erythroid stimulants (epoetin [Procrit] and darbepoetin
{Aranesp]. Suboptimal doses of erythropoietic products can lead to uncorrected anemia, and doses
above the desired range can lead to unnecessary adverse events such as thrombosis and cardiovascular
events. Either instance could potentially lead to poor clinical outcomes and excessive payer expense.
Typically, for Anemia of CKD origin weekly doses greater than 100mcg of Aranesp or 34,000 units of
Procrit should be verified for appropriateness. In addition, in order for the erythroid stimulant agents to be
effective, iron stores must be replenished and therefore, either oral or parenteral iron must be given. BZ
REGULATORY UPDATE
Copyright 2006
All Rights Reserved
Published by Omnicare, Inc.
distributed by PBM Plus, Inc.
Page - 6
HEALTHLINE
October 2006
Dispensing Controlled Substances for Pain Treatment
The Drug Enforcement Administration (DEA) has issued a proposed rule that will allow a physician to
prescribe up to a 90-day supply of Schedule II controlled substances during a single office visit, where
medically appropriate. This will make it easier for patients with chronic pain or other chronic conditions, to
avoid multiple trips to a physician. The Notice of Proposed Rulemaking is accompanied by a policy
statement, “Dispensing Controlled Substances for the Treatment of Pain,” which provides information
requested by medical professionals regarding DEA’s position on this important issue. A sixty-day public
comment period on the Notice of Proposed Rulemaking began on September 6, 2006, the date of
publication. For additional information please go to www.dea.gov.
Government to Study Impact of Part D Implementation in Nursing Homes
The HHS Office of Inspector General (OIG) will be conducting a review of the recent implementation of
Medicare Part D for dual eligible nursing home residents. This study is being conducted to determine the
impact that the implementation of Part D had on patients and staff, and is designed to identify the
strengths and weaknesses of the program. As part of their review, the OIG will be conducting telephone
interviews with administrators and medical directors from randomly selected facilities, which will be
notified by letter. Participation in the interviews is encouraged to ensure that the OIG has accurate
information on the how Part D implementation impacts residents in nursing facilities.
New Medicare Learning Network Products
The new CMS “Website Wheel” is now available. This resource provides up-to-date web addresses for
the most frequently used Medicare provider web pages, including the new URLs that resulted from the
CMS Website redesign. You can request a copy of the CMS Website Wheel, free of charge, by going to
the MLN roder page: http://cms.meridianksi.com/kc/main/kc_frame.asp?kc_ident=kc0001&loc=5. KM
Revised Guidance for Long-Term Care Surveyors
Revised guidance for long-term care surveyors regarding Unnecessary Drugs, Pharmacy Services, Drug
Regimen Review and Labeling and Storage of Drugs and Biologicals will be effective December 18, 2006.
These changes will be summarized in the November issue of HealthLines. SK
Contributing Authors for This Issue:
Susan J. Klem, BS Pharm., CGP, FASCP
Regional Clinical Director
Great Lakes and Great Plains Regions
Kelli Marsh, RHIA, RAC-C
Vice President of Support Services
Omnicare Pharmacies of Northern and Central Ohio
David Pregizer, RPh
Consultant Pharmacist
Heartland Pharmacy of Pennsylvania
Barbara J. Zarowitz, PharmD, CGP, BCPS, FCCP
Chief Clinical Officer,
Vice President of Professional Services
Copyright 2006
All Rights Reserved
Published by Omnicare, Inc.
distributed by PBM Plus, Inc.
Page - 7
HEALTHLINE
October 2006
Editorial Board
Karen Burton, R. Ph., GCP, FASCP
Mark Coggins, Pharm. D., GCP, FASCP
Kelly Hollenack, Pharm. D. CGP
Philip King, Pharm. D., GCP, FASCP
Susan Kleim, B.S., Pharm., GCP, FASCP
Terry O’Shea, Pharm. D., GCP, FASCP
Elmer Schmidt, Pharm. D., GCP, FASCP
Barbara J. Zarowitz, Pharm. D., GCP, FASCP
Copyright 2006
All Rights Reserved
Published by Omnicare, Inc.
distributed by PBM Plus, Inc.
Page - 8