* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download draft cover letter to science
Extinction debt wikipedia , lookup
Overexploitation wikipedia , lookup
Island restoration wikipedia , lookup
Biodiversity wikipedia , lookup
Biogeography wikipedia , lookup
Latitudinal gradients in species diversity wikipedia , lookup
Decline in amphibian populations wikipedia , lookup
Operation Wallacea wikipedia , lookup
Restoration ecology wikipedia , lookup
Great American Interchange wikipedia , lookup
Biodiversity action plan wikipedia , lookup
Holocene extinction wikipedia , lookup
Conservation movement wikipedia , lookup
Conservation psychology wikipedia , lookup
Molecular ecology wikipedia , lookup
Conservation biology wikipedia , lookup
Reconciliation ecology wikipedia , lookup
Theoretical ecology wikipedia , lookup
Ecological fitting wikipedia , lookup
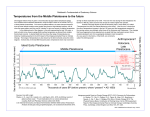
DRAFT COVER LETTER TO SCIENCE We are pleased to submit the manuscript Pleistocene Rewilding: An Optimistic Vision for 21st Century Conservation. In this review article, we justify using extant conspecifics and related species as functional analogs of recently extinct North American vertebrates to partially restore ecological roles and evolutionary potential that were lost with the end-Pleistocene megafaunal extinction. Pleistocene rewilding, conceived as a series of carefully managed ecosystem manipulations, would counter the pest-and-weed biotas promoted by human impact, facilitate the persistence and evolution of large vertebrates on a global scale, and change the underlying premise of conservation from managing extinction to actively restoring biological processes. Our vision is supported by ecological, economic, esthetic, ethical, and evolutionary considerations. Because of the twelve author’s unusually diverse experiences, expertise, and pre-existing biases regarding Pleistocene rewilding, we sought pre-publication outside reviews from only four people: S. Dobrott, M. Phillips, and J. C. Truett of the Turner Endangered Species Program, and C. Vriesendorp, a conservation biologist at the Field Museum of Natural History. None of the material has been published or under review elsewhere. We recognize that our paper will almost certainly be highly provocative, and we are confident that R. K. Colwell and D. J. Erwin of your board of editors are highly qualified to give our manuscript a fair and scholarly consideration. Suggestions for external reviewers are below. David Western Email: [email protected] Email: [email protected] Wildlife Conservation Society & African Conservation Centre, Box 62844, Nairobi, Kenya James H. Brown Email: [email protected], Biology Department University of New Mexico Albuquerque, NM 87131 Terry Chapin Email: [email protected] Department of Biology and Wildlife Institute of Arctic Biology, University of Alaska Fairbanks, Alaska, 99775 Paul K. Dayton Email: [email protected] Scripps Institution of Oceanography, UCSD 9500 Gilman Drive La Jolla CA, 92093-0227 Tim Flannery Email: [email protected] South Australia Museum, North Terrace Adelaide, South Australia 5000, Australia Jeremy Jackson Email: [email protected] Scripps Institution of Oceanography, UCSD 9500 Gilman Drive La Jolla CA, 92093-0244 For the authors, C. Josh Donlan Department of Ecology and Evolutionary Biology, Corson Hall, Cornell University Ithaca, NY 14853 U.S.A. [email protected] Voice: 607.254.4269 Voice: 607.227.9768 Fax: 607.255.8088 Pleistocene Rewilding: An Optimistic Vision for 21st Century Conservation C. Josh Donlan1,*, , Joel Berger2, Carl E. Bock3, Jane H. Bock3, David A. Burney4, James A. Estes5, Dave Foreman6, Paul S. Martin7, Gary W. Roemer8, Felisa A. Smith9, Michael E. Soulé10, and Harry W. Greene1 Running Head: 21st century conservation Word Count: 4860 1 Department of Ecology and Evolutionary Biology, Cornell University, Ithaca, NY 14853 U.S.A. 2 Teton Field Office, North American Program, Wildlife Conservation Society, Moose, WY 83012 U.S.A. 3 Department of Ecology and Evolutionary Biology, University of Colorado, Boulder, CO 80309 U.S.A. 4 Department of Biological Sciences, Fordham University, Bronx, NY 10458 U.S.A. and National Tropical Botanical Garden, Kalaheo, HI 96741 U.S.A 5 U.S. Geological Survey, University of California, Santa Cruz, CA 95060, U.S.A. 6 The Rewilding Institute, P.O. Box 13768, Albuquerque, NM 87192 U.S.A. 7 Desert Laboratory, Department of Geosciences, University of Arizona, Tucson, AZ 85721 U.S.A. 8 Department of Fishery and Wildlife Sciences, New Mexico State University, Las Cruces, NM 88003 U.S.A. 9 Department of Biology, University of New Mexico, Albuquerque, NM 87131l U.S.A. 10 P.O. Box 2010, Hotchkiss, CO 81419 U.S.A. *Correspondence: Department of Ecology and Evolutionary Biology Corson Hall, Cornell University Ithaca, NY 14853 U.S.A. [email protected] Voice: 607.254.4269 Voice: 607.227.9768 Fax: 607.255.8088 1 We propose to partially restore ecological functionality and evolutionary potential to North America that were lost with the late Pleistocene megafaunal extinction. From Bolson tortoises (Gopherus flavomarginatus) and feral equids to Asian elephants (Elephas maximus) and Holarctic lions (Panthera leo), our vision for this restoration begins immediately and spans the coming century. We justify using extant conspecifics and related species as functional analogs of recently extinct vertebrates on ecological, evolutionary, economic, esthetic, and ethical grounds. Pleistocene rewilding, conceived as a series of carefully managed ecosystem manipulations, would counter the pest-and-weed biotas promoted ever more widely by human impact, facilitate the persistence and evolutionary potential of large vertebrates on a global scale, and change the underlying premise of conservation from managing extinction to actively restoring ecological and evolutionary processes. 2 Africa’s large mammals are dying (1-4), stranded on a continent where wars are being fought over scarce resources (5). With the loss of most large mammals and their commensals, much of North America died c. 13,000 years ago (6-8). More than any other species in the history of life, humans cause extinctions, change ecosystems, and affect the very future of evolution (9-13), and they surely will continue to do so by default or design (14). Here, we outline an alternative vision for 21st century conservation biology that is based on our late Pleistocene heritage, one that is bold, optimistic, and ethically defensible. There has been no single, non-arbitrary conservation benchmark since the end of that epoch. Thus, we propose Pleistocene rewilding—re-instituting ecologically and evolutionary processes that were transformed or eliminated by megafaunal extinctions— as a conservation priority in North America. We first discuss the ecological, evolutionary, economic, esthetic, and ethical justifications for this proposition, then describe six case histories to inform the debate we aim to provoke. Our vision is based on the following observations. First, Earth is nowhere pristine in the sense of being substantially free from human influence (1-7,9-15). Human economics, politics, demographics, and chemicals pervade every ecosystem; even our largest parks require management and suffer extinction (16). These human impacts are unprecedented in their magnitude, cosmopolitan in their distribution, and show alarming signs of worsening (9,10,12-14). Second, conservation biology is largely characterized as doom and gloom; conservationists have largely accepted this losing battle to slow biodiversity loss, and with few exceptions struggled only to diminish its rate. Third, future human demographic and land use patterns will be dynamic and uncertain. Many areas in the U.S., such as parts of the Great Plains, are depopulating (17), and may offer 3 future conservation opportunities (18). Fourth, humans probably were responsible to some significant degree for the late Pleistocene extinctions in North America (6,7) and our subsequent activities have curtailed survival prospects and evolutionary potential for most large vertebrates (19). We therefore bear an ethical responsibility, as citizens and scientists, to redress these problems insofar as possible. JUSTIFICATIONS FOR PLEISTOCENE REWILDING IN NORTH AMERICA Our prevalent conservation benchmarks dictate which taxa are regarded as native and which are not, irrespective of ecological and historical insights (20). In North America we routinely turn to Columbus and 1492 as a de facto restoration baseline (21), thereby discounting significant, earlier ecological impacts by humans (22,23). The arrival of the first Americans, the Clovis culture (c. XX,XXX YBP, 24), constitutes a less arbitrary benchmark that is justifiable from multiple perspectives. Prior to the late Pleistocene extinctions, mammal body size distributions were remarkably similar across all continents, despite little overlap in species composition (25), and the subsequent extinction of most large mammals in Australia and the Americas drastically altered those distributions to favor smaller forms (Fig. 1, 26). Given that body size appears to be highly conserved across taxa (25), these losses are significant with respect to ecology and vertebrate evolutionary potential, particularly in the Americas where the losses were greatest (8,27,28) Ecology and conservation have recently become more process-oriented (30). Prehistoric, historic, and contemporary evidence lend credence to the premise large carnivores and herbivores often play important ecological roles in the maintenance of biodiversity (8,27,29-35). It follows that many now extinct large mammals must have 4 shaped the life histories of extant species through the selective forces of strong species interactions (8,27,35,36). In some cases interaction loss leads to extinction (37,38), others result in disequilibria (8,39,40). Numerous North American species are now believed to be anachronistic on modern landscapes due to losses of species interactions c. 13,000 YBP (41). Osage orange (Maclura pomifera) and American pronghorn (Antilocapra americana) provide two notable examples – extinct proboscidians and other large herbivores likely dispersed seeds of the former and the American cheetah (Acinonyx jubatus) likely preyed on the latter (8,36,41). The inferred ecological roles of Pleistocene megafauna and their modern conspecifics or analogs imply specific hypotheses that would be tested as prerequisites during the stages of rewilding described below. While evolutionary perspectives have been raised in conservation planning (42,43), the bold actions needed to preserve evolutionary potential in the wake of the drastic decline in biodisparity have not been addressed. Must we accept the end of speciation of large vertebrates (19), or shall we take responsibility for partially restoring that potential? Africa and parts of Asia are now the only land-based places where the Pleistocene megafauna remain intact, and the loss of many of these species within this century seems likely (1,3,4,13,44,45). Given the demonstrable extinction risks for the Earth’s remaining megafauna and the possibility that North American sites could serve as additional refuge and help preserve evolutionary potential, regional rewilding (46,47) carries global conservation implications. Humans have an emotional relationship with large predators and herbivores that extends back into the Pleistocene (48). A public understanding of ecological and evolutionary history, inspired by tangible esthetic experiences, would strengthen overall 5 support for the conservation of biodiversity and wilderness (49). More than 1.5 million people annually visit San Diego Zoo’s Wild Animal Park to catch a glimpse of large mammals, exceeding visitation for 80% of U.S National Parks (50). Per capita visitation to U.S. National Parks has been declining since 1987—the first time since the 1930s (51). Pleistocene rewilding would bring timely economic incentives to both private and public lands. CASE STUDIES: FROM THE TORTOISE TO THE LION The Bolson tortoise (Gopherus flavomarginatus) was probably widely distributed across the Chihuahuan Desert until the late Pleistocene (52). Today, it is critically endangered and restricted to a small area in north-central Mexico. Once part of the North American megafauna, weighing up to 50 kg, and susceptible to human overkill, the Bolson tortoise disappeared from more than 90% of its range by the end of the Pleistocene (53). A number of appropriate sites exist for reintroduction, including Big Bend National Park and large private ranches in the Southwest. Along with clear conservation benefits for the species, tortoise reintroductions may increase local biodiversity by adding heterogeneity to the landscape via burrow construction, as has been documented for other Gopherus species (54). Repatriating the continent’s largest surviving temperate terrestrial reptile could precipitate a variety of ecological, evolutionary, economic, and cultural benefits, with no apparent costs (Table 1, Fig. 2). Feral equids (Equus caballus, E. asinus) have been abundant in North America since they were introduced by Europeans 500 years ago (55). From an evolutionary and ecological perspective, horses are native to North America: they were present there for 6 most of the last 50 million years, radiated from that continent, and were diverse on it until the late Pleistocene (Table 1,56). Feral horses and burros are widely viewed as ecological pests, but in the context of historical ecology they are plausible analogs for extinct equids (35). Although the ecological role and impacts of feral horses are variable over temporal and spatial scales (55,57-59), they disperse large-seeded plants and thus may compensate for certain large Pleistocene mammals now absent in North America (8). Wild asses (E. hemionus) and Przewalski’s horse (E. przewalskii) are critically endangered or extinct in the wild (60), so free-roaming North American populations would help curtail extinction and repatriate equids to their evolutionary homeland. Whether the overall impact of rewilding with equids would be positive or negative in local ecological and economic contexts might depend on temporal and spatial dynamics, and thus perhaps on the presence of appropriate predators. The center of camelid evolution was North America, where four species of camels and llamas were present in the late Pleistocene (61,62). Only four species remain today worldwide, restricted to the Gobi desert and South America. Wild Bactrian camels (Camelus bactrianus) are on the verge of extinction, less than 1000 animals remain (63). Domesticated or captive Bactrian camels could be reintroduced to parts of North America, further assuring the evolutionary potential of camelids and serving as browser analogs for the closely related Pleistocene Camelops (64). Camels potentially offer biodiversity benefits to arid and semi-arid North American ecosystems by browsing on woody species that now often form homogeneous landscapes (65). In the 1850s, when Lt. Beale led the Camel Military Corps from Texas to California, his animals browsed on creosote (Larrea tridentata) and other brush species that today often dominate 7 overgrazed desert lands (66). Camels might bring economic benefits as well; in Australia, well-managed co-grazing programs of cattle and camels produce additional markets for meat, milk, and fiber without negatively impacting the landscape or cattle production (67). The American cheetah was the likely principal agent of selection for the pronghorn, whose astounding speed evolved in the context of four million years of predation on North American grasslands (36). The African cheetah (A. jubatus), a close relative of the American cheetah (68), was once found throughout Africa and southwestern Asia; its current distribution has been greatly reduced and it has only a modest chance of persisting in the wild into the next century (44,69). Breeding programs are not self-sustaining and wild populations have continued to sustain captive ones (68). Nonetheless, some of the over 1,000 animals in captivity (69,70) could replace the North American cheetah as ecological and evolutionary analogs. Conservation scenarios for cheetah are unique in that the majority of the remaining individuals are located outside of protected areas, commonly on commercial livestock and game farmland (69,71,72). While farmers often perceive cheetahs as threats and persecute them, environmental education and alternative pastoral practices have recently proved useful in promoting their coexistence with humans (71). Cheetah populations in the southwest United States would restore what must have been strong interactions with pronghorn, help save the world’s fastest species from extinction, and facilitate economic alternatives to ranchers through participation in ecotourism. Five species of proboscidians roamed North America in the Pleistocene (61,62). Elephants (Loxodonta africana) play keystone roles on the African landscape (33,73), as 8 proboscidians likely once did in the Americas (8). African elephants prevent woodland regeneration and promote grasslands; elevated densities appear to be the primary driver of woodland loss (75,76). Encroachment of woody and shrub plant species over the past century now threaten the arid grasslands of western North America (65), and while the causes are complex and debated (65,74), browsing elephants could counter shrub and tree (e.g., Juniperus) invasion and increase landscape heterogeneity. Managed elephant populations in North America could thus enhance biodiversity and economically benefit ranchers through grassland maintenance and ecotourism. Further, many elephant populations, particularly in Asia and West Africa, are in grave danger of extinction (45), and captive populations are not sustainable for either species (77,78). With the appropriate resources and vision, captive stock and some of the 16,000 domesticated elephants in Asia (79) could contribute to the wild future for this flagship species by initiating a North American population. Fencing, which has proven effective in mediating human-elephant conflict in Africa (80), would be the main economic cost. Lions, which prey on wild equids and other ungulates, represent the ultimate vision for Pleistocene rewilding. The American lion (Panthera leo atrox) was conspecific with surviving African and Asian lions, and they may collectively have once been the most wide-ranging wild land mammal of all time (62). Lions play a pivotal role in the Serengeti ecosystem, along with other predators, in regulating prey populations (34), as they likely once did in the Americas and Eurasia. African lions are increasingly threatened by habitat destruction and disease, and the Asiatic lion (P. leo persica) is now critically endangered, with a single population in the Gir Forest of India (4,81,82). Establishing additional populations is vital for their long-term persistence; recent 9 attempts in India have failed (81). Given public attraction to large predators, the potential esthetic and economic benefits of reintroducing lions to North America are obvious. BUMPS ALONG THE ROAD TO PLEISTOCENE REWILDING Potentially serious and legitimate objections to Pleistocene rewilding must be faced candidly, with all available information and within the above-mentioned ecological, evolutionary, economic, esthetic, and ethical contexts . With respect to genetics, the megafaunal proxies we propose are not literally the same animals lost in the late Pleistocene. The African lion and cheetah are somewhat smaller than their extinct American counterparts, Camelus is different from Camelops, and so forth. “Same” is a relative concept, however, as illustrated by the peregrine falcon (Falco peregrinus). Celebrated as one of the single largest successful conservation efforts (83), the North American peregrine program relied on large numbers of captive-bred birds from seven subspecies, obtained from four continents (84,85). Despite morphological and ecological variation among the founders, there were no differences among subspecies in breeding success of the reintroduced birds (85), and they now serve as a collective analog for the midwestern peregrine population that went extinct in the 1960s. Is a pre-1492 benchmark unrealistic in our current world? Another recent endangered species program contradicts objections that bringing back megafauna would waste precious conservation dollars, since at least initially their population would not be self-sustaining. The California condor (Gymnogyps californianus) was present throughout North America until the late Pleistocene, disappearing along with the megafauna upon which it fed, and persisting only in Pacific coastal regions. Condors last 10 roamed over the Grand Canyon c. 10,000 years ago, and scavenged on now extinct taxa, including mammoths (Mammuthus sp), horses, and camels (86,87). Nevertheless, condors from a captive breeding program now soar over Colorado River canyons and rely on cattle carcasses as food subsidies. While this program is unsustainable without active management, few would now argue against efforts to save and diversify condor populations (88). Should we not also return some of the large herbivores and their carnivores that collectively once fed these giant scavengers? Other potentially serious objections to Pleistocene rewilding include the possibility of catastrophic disease transmission, the fact that habitats have not remained static since the end of the Pleistocene, and unexpected ecological and social consequences of species introductions (89,90). These are problems that must be addressed in advance by sound research and management plans for each species on a case-by-case, locality-by-locality basis. Well-designed, hypothesis-driven field experiments will be needed to assess the impacts of potential introductions before releases take place, especially if removal would prove difficult should unintended problems arise. Predators offer unique challenges for conservation that must be mitigated with protective measures and attitudinal adjustments. Just as is the case in African parks today, we must accept the necessity for precautionary behavior in truly wild areas, as well as the fact that carnivores kill and consume other creatures. WHERE TO BEGIN? We envision several stages to Pleistocene rewilding, with the first already underway. Equids, camelids, and other ungulates are increasingly prevalent on western landscapes, and there is widespread discussion of a buffalo commons in the Great Plains 11 (18). Translocation of a captive population of Bolson tortoises to a private ranch in New Mexico is currently under study. Small-scale experiments are urgently needed to assess the economic, ecological, and cultural implications of more widespread re-introductions of these and other herbivores. Large-tracts of private and public lands in the southwest United States (91) are potentially appropriate for Pleistocene rewilding, with the fossil record and carefully designed research as guideposts and safeguards. Private lands likely hold the most immediate potential: more than 77,000 Asian and African large mammals (71 species) now roam free on Texas ranches (92), although their significance for conservation remains largely unevaluated. A second stage can also begin immediately, with the experimental maintenance of small numbers of cheetah, lions, and elephants on managed private property, such that their ecological impact and sustainability can be carefully studied. The requisite animals are already present in the U.S. or can be readily produced by captive breeding; the primary logistical innovation at this point is to provide them with naturalistic selective regimes, including predator-prey relationships among herbivores and carnivores. As with other organisms targeted for rewilding, the details of planning and management for each species should be handled by expert groups from a variety of pertinent disciplines. The third and more distant stage would include an enormous Ecological History Park, encompassing thousands of square miles in what are already economically depressed parts of the Great Plains (18). Secure game fencing would limit the movements of free-living ungulates, elephants, and large carnivores, including lions. As in Africa today, surrounding towns would derive their livelihoods from land management and tourism related jobs. The initiation and precise nature of each of these stages would 12 depend on information derived from previous efforts, such that risks would be identified and negative effects minimized. Two prerequisites of critical importance are rigid adherence to established protocols, including specification of goal criteria and monitoring regimes, and incentives for local land owners and other stakeholders. In the coming century, we will decide, by default or design, on the extent to which our world incorporates other species. The default scenario will surely include ever more pest-and-weed dominated landscapes, the global loss of large vertebrates, and a continuing struggle to slow the loss of biodiversity. Pleistocene rewilding is an optimistic alternative vision that scales globally to other continents and oceanic islands (93-96). To those who find the objections to Pleistocene rewilding compelling, we ask, are you content with the negative slope of our current conservation philosophy? Are you willing to risk the extinction of the remaining megafauna should economic, political, and climate change prove catastrophic for Bolson tortoises, cheetah, camelids, lions, elephants and other species within their current ranges? Are you willing to settle for an American wilderness that is severely depauperate relative to just 100 centuries ago? The obstacles to Pleistocene rewilding are indeed substantial and the risks are not trivial, but we can no longer accept a hands-off approach to wilderness preservation as realistic or defensible. It is time to not only save wild places, but to rewild and reinvigorate them. 13 REFERENCES AND NOTES 1. A. Balmford et al., Science 291, 2616 (2001). 2. G. Vogel, Science 287, 2386 (2000). 3. C. J. M. Musters, H. J. de Graaf, W. J. ter Keurs, Science 287, 1759 (2000). 4. J. Marchant, New Scientist 14, 37 (2001). 5. J. Diamond, Collapse: How Societies Choose to Fail or Succeed (Viking, New York, 2004). 6. P. S. Martin, R. G. Klein, Eds., Quaternary Extinctions: A Prehistoric Revolution (University of Arizona Press, Tucson, 1984). 7. A. D. Barnosky, P. L. Koch, R. S. Feranec, S. L. Wing, A. B. Shabel, Science 306, 70 (2004). 8. D. H. Janzen, P. S. Martin, Science 215, 19 (1982). 9. H. J. Smith, Science 302, 1171 (2003). 10. O. E. Sala et al., Science 287, 1770 (2000). 11. N. Myers, A. H. Knoll, Proc. Natl. Acad. Sci. U. S. A. 98, 5389 (2001). 12. J. A. Thomas et al., Science 303, 1879 (2004). 13 C. D. Thomas et al., Nature 427, 145 (2004). 14. D. Western, Proc. Natl. Acad. Sci. U. S. A. 98, 5458 (2001). 15. P. Vitousek, H. A. Mooney, J. Lubchenco, J. M. Melilo, Science 277, 494 (1997). 16. W. D. Newmark, Nature 325, 430 (1987). 17. R. E. Lonsdale, J. C. Archer, J. Geogr. 97, 108 (1998). 18. D. E. Popper, F. J. Popper, Geogr. Rev. 89, 491 (1999). 14 19. M. E. Soulé, in Conservation Biology: An Evolutionary-Ecological Perspective M. E. Soulé, B. A. Wilcox, Eds. (Sinauer, Sunderland, 1980) pp. 151-169. 20. C. J. Donlan, P. S. Martin, Conserv. Biol. 18, 267 (2004). 21. A. S. Leopold, S. A. Cain, C. M. Cottam, I. N. Gabrielson, T. L. Kimball, Transactions of the Twenty-eight North American Wildlife and Natural Resources Conference 28, 29 (1963). 22. C. Kay, R. T. Simmons, Eds., Wilderness and Political Ecology: Aboriginal Influences and the Original State of Nature (University of Utah Press, Salt Lake City, 2002). 23. P. S. Martin, C. R. Szuter, Conserv. Biol. 13, 36 (1999). 24. G. Haynes, The Early Settlement of North America: the Clovis Era (Cambridge University Press, Cambridge, 2002). 25. F. A. Smith et al., American Naturalist 163, 672 (2004). 26. S. K. Lyons, F. A. Smith, J. H. Brown, Evolutionary Ecology Research 6, 339 (2004). 27. C. J. Donlan, P. S. Martin, G. W. Roemer, in Whales, Whaling, and Ocean Ecosystems J. A. Estes, R. L. Brownell, D. P. DeMaster, D. F. Doak, T. M. Williams, Eds. (University of California Press, Berkley, in press). 28. A. Purvis, P.-M. Agapow, J. L. Gittleman, G. M. Mace, Science 288, 328 (2000). 29. J. Terborgh et al., in Continental Conservation: Scientific Foundations of Regional Reserve Networks M. E. Soulé, J. Terborgh, Eds. (Island Press, Washington, D.C., 1999) pp. 39-64. 30. M. E. Soulé, J. A. Estes, J. Berger, C. M. Del Rio, Conserv. Biol. 17, 1238 (2003). 15 31. J. A. Estes, M. T. Tinker, T. M. Williams, D. F. Doak, Science 282, 473 (1998). 32. J. Berger, P. B. Stacey, L. Bellis, M. P. Johnson, Ecological Applications 11, 947 (2001). 33. R. N. Owen-Smith, Megaherbivores: the Influence of Very Large Body Size on Ecology (Cambridge University Press, Cambridge, 1988) 34. A. R. E. Sinclair, S. Mduma, J. S. Brashares, Nature 425, 288 (2003). 35. S. A. Zimov et al., American Naturalist 146, 765 (1995). 36. J. A. Byers, American Pronghorn: Social Adaptations and the Ghosts of Predators Past (University of Chicago, Chicago, 1997). 37. L. P. Koh et al., Science 305, 1632 (2004). 38. P. D. Steinberg, J. A. Estes, F. C. Winter, Proc. Natl. Acad. Sci. U. S. A. 92, 8145 (1995). 39. J. B. C. Jackson, Coral Reefs 16, S23 (1997). 40. J. M. Pandolfi et al., Science 301, 955 (2003). 41. C. C. Barlow, The Ghosts of Evolution: Nonsensical Fruit, Missing Partners, and Other Ecological Anachronisms (Basic Books, New York, 2000). 42. T. L. Erwin, Science 253, 750 (1991). 43. O. H. Frankel, M. E. Soulé, Conservation and evolution (Cambridge University Press, Cambridge; New York, 1981). 44. P. M. Gros, Biological Conservation 106, 177 (2002). 45. S. Blake, S. Hedges, Conserv. Biol. 18, 1191 (2004). 46. M. Soulé, R. Noss, Wild Earth 8, 18 (1998). 16 47. D. Foreman, Rewilding North America: a Vision for Conservation in the 21st Century (Island Press, Washington D.C., 2004). 48. P. Shepard, Coming Home to the Pleistocene (Island Press, Washington D.C., 1998). 49. H. W. Greene, Trends Ecol. Evol. 20, 23 (2005). 50. From 1999-2004, San Diego Zoo’s Wildlife Animal Park received over 1.5 million visitors a year (C. Simmons, personal communication). Twelve of 53 U.S. National Parks received over 1.5 million visitors in 2000 (National Park Service. 2000. National Park Service Statistical Abstract. Public Use Statistics Office. Denver, Colorado). 51. O. R. W. Pergams, B. Czech, J. C. Haney, D. Nyberg, Conserv. Biol., 1617 (2004). 52. R. B. Bury, D. J. Morafka, C. J. McCoy,, Ann. Carnegie Mus. 57, 5 (1988). 53. Harsh winters could have also played a contributing role [T. R. Van Devender et al., Herpetologica 32, 298 (1976); K. B. Moodie. T. R.Van Devender, Herpetologica 35, 198 (1979)]. However, G. flavomarginatus is relatively cold-tolerant, evidenced by 20+ year survival of a reproducing captive population in Arizona [A. Appleton, Desert Tortoise Council Symposium Proceedings, 164 (1978). Chelonians and their eggs were part of the exploited megafauna in the American Southwest and elsewhere [K. B. Moodie. T. R.Van Devender, Herpetologica 35, 198 (1979); M. C. Stiner et al. Science, 283, 190 (1999); R. W. Tayor Jr., Bulletin of the Florida State Museum, Biological Sciences, 28, 79 (1982)]. 54. S. A. Kaczor, D. C. Hartnett, Am. Midl. Nat. 123, 100 (1990). 17 55. J. Berger, Wild Horses of the Great Basin: Social Competition and Population Size (University of Chicago Press, Chicago, 1986). 56. B. J. MacFadden, Fossil horses: Systematics, Paleobiology and Evolution of the Family Equidae (Cambridge University Press, Cambridge, 1992). 57. C. Menard, P. Duncan, G. Fleurance, J. Y. Georges, M. Lila, J. of Appl. Ecol. 39, 120 (2002). 58. P. S. Levin, J. Ellis, R. Petrik, M. E. Hay, Conserv. Biol. 16, 1364 (2002). 59. E. A. Beaver, P. F. Brussard, J. Arid Environ. 59, 271 (2004). 60. P. D. Moehlman, Ed., Equids: Zebras, Asses, and Horses: Status Survey and Conservation Action Plan. (IUCN/SCC Equid Specialist Group, IUCN Gland, Switzerland, 2002). 61. F. A. Smith et al., Ecology 84, 3403 (2003). 62. B. Kurtén, E. Anderson, Pleistocene Mammals of North America (Columbia University Press, New York, 1980). 63. J. Hare, in IUCN 2003 Red List of Threatened Species IUCN, Ed. (2001). 64. S. D. Webb, Bulletin of the Los Angeles County Museum, Science 1, 1 (1965). 65. O. W. Van Auken, Annu. Rev. Ecol. Syst. 31, 197 (2000). 66. P. S. Martin, Bioscience 20, 218 (1970). 67. A. Phillips, J. Heucke, B. Dorgers, G. O'Reilly, Co-grazing Cattle and Camels. A Report for the Rural Industries Research and Development Corporation (Rural Industries Research and Development Corporation, Kingston, Australia, 2001). 68. D. B. Adams, Science 205, 1155 (1979). 18 69. T. M. Caro, Cheetahs of the Serengeti plains: Group Living in an Asocial Species (University of Chicago Press, Chicago, 1994). 70. L. Marker-Kraus, International Zoo Yearbook 35, 27 (1997). 71. L. L. Marker, M. G. L. Mills, D. W. Macdonald, Conserv. Biol. 17, 1290 (2003). 72. Ninety-five percent of Namibia's cheetahs live on commercial livestock farmland, where lions (Panthera leo) and spotted hyaenas (Crocuta crocuta) have been eliminated [L. L. Marker, thesis, Univ. of Oxford (2002)]. 73. H. T. Dublin, in Serengeti II: Dynamics, Management, and Conservation of an Ecosystem A. R. E. Sinclair, P. Arcese, Eds. (University of Chicago, Chicago, 1995) pp. 71-90. 74. J. H. Brown, T. J. Valone, C. G. Curtin, Proc. Natl. Acad. Sci. U. S. A. 94, 9729 (1997). 75. D. Western, D. Maitumo, Afr. J. Ecol. 42, 111 (2004). 76. I. J. Whyte, R. v. Aarde, S. L. Pimm, in The Kruger Experience: Ecology and Management of Savanna Heterogeneity J. T. Du Toit, H. C. Biggs, K. H. Rogers, Eds. (Island Press, Washington D.C., 2003) pp. 332-348. 77. R. J. Wiese, Zoo Biol. 19, 299 (2000). 78. D. Olson, R. J. Wiese, Zoo Biol. 19, 311 (2000). 79. R. C. Lair, Gone Astray: the Care and Management of the Asian elephant in Domesticity (Food and Agriculture Organization of the United Nations, Bangkok, 1997). 80. R. E. Hoare, Pachyderm 19, 54 (1995). 81. K. Nowell, P. Jackson, Eds., Wild Cats. Status Survey and Conservation Action Plan. (IUCN/SSC Cat Specialist Group. IUCN, Gland, Switzerland, 1996). 19 82. M. Roelke-Parker et al., Nature 379, 441 (1996). 83. T. J. Cade, W. Burnham, Eds., Return of the Peregrine: A North American Saga of Tenacity and Teamwork (The Peregrine Foundation, Boise, 2003). 84. Because of a lack of genetically more appropriate founders, parents of peregrine falcons that were released into eastern and midwest U.S. and parts of Canada came from captive stock from North America, Europe, South America, and Australia, a total over approximately 2500 birds. See ref. 85. 85. H. B. Tordoff, P. T. Redig, Conserv. Biol. 15, 528 (2001). 86. S. D. Emslie, Science 237, 768 (1987). 87. The condor ranged throughout North America, including Texas, Arizona, and New Mexico in the late Pleistocene. At the time of European arrival, condors occurred only along a narrow Pacific coastal strip. Controversial evidence suggests that condors returned briefly to the southwest U.S. in the 1700s in response to the introduction of domesticated cattle, horses, and sheep. [ref 86; Federal Register, 61, 201 (1996); S. Emslie, pers. comm.; L. Kiff, pers. comm.]. 88. N. Snyder, H. Snyder, The California Condor: a Saga of Natural History and Conservation, (Academic Press, San Diego, California, 2000). 89. G. W. Roemer, C. J. Donlan, F. Courchamp, Proc. Natl. Acad. Sci. U. S. A. 99, 791 (2002). 90. P. Dazak, A. A. Cunningham, A. D. Hyatt, Science 287, 443 (2000). 91. R. A. Mittermeier et al., Proc. Natl. Acad. Sci. U. S. A 100, 10309 (2003). 92. D. J. Schmidly, Texas Natural History: A Century of Change (Texas Tech University Press, Lubbock, 2002). 20 93. P. S. Martin, D. A. Burney, Wild Earth Spring, 57 (1999). 94. D. W. Steadman, P. S. Martin, Earth-Sci. Rev. 61, 133 (2003). 95. R. Stone, Science 282, 31 (1998). 96. T. Flannery, The Eternal Frontier (Atlantic Monthly Press, New York, 2001). 97. We thank Environmental Leadership Foundation, Lichen Foundation, Turner Endangered Species Fund, New Mexico State University, and Ladder Ranch for financially and logistically supporting the workshop on which this paper is based. Tom Gorton and Transmutations/S.T.F. assisted with the figures. We are especially grateful to S. Dobrott, M. K. Phillips, and J. C. Truett for their hospitality at the Ladder Ranch, workshop participation, and constructive criticisms of this manuscript. 21 TABLE 1. The magnitude of biodiversity loss of North America megafauna and potential benefits and costs of Pleistocene rewilding (+ represents an increase in respective category). Late Pleistocene (LP) and current diversity of continental North American largebodied mammals, along with potential species proxies. Order or Family LP Current 1 (T/E) Proxy 13 8 (3) Cheetah predation Ecological Costs Economic Benefits Economic Costs 3 ? tourism fencing; livestock 4 mortality? ++ +++ Lion predation ? tourism; hunting human conflict ++ +++ Equids seed 5 dispersal;prey ? tourism fencing; compete with cattle +++ ++ fencing + +++ fencing +++ ++ none +++ + 2 Ecological Benefits Ease of Popularity Establishment Predators Felidae Ursidae 6 3 (2) Canidae 9 7 (1) Xenarthra 14 6 (2) Bovidae 13 5 (2) Equidae 11 0 Cervidae 10 5 Antilocapridae 6 1 Proboscidea 5 0 Elephants Camelidae 4 0 Camels Tapiridae 4 1 Tayassuidae 3 1 Hydrochoeridae 2 0 Castoridae 2 1 Testudinidae ? 0 102 38 (10) Herbivores Total 1 Bolson tortoise heterogeneity; density & scale 6 seed dispersal dependent effects heterogeneity; potential 7 seed dispersal overbrowsing heterogeneity 8 none / slight tourism; hunting meat, fiber production tourism Extant species in each taxa are significantly biased towards smaller body size (ref 21). T/E = threatened or endangered, listed by U.S. Endangered Species Act or 2001 IUCN category Near Threatened (or equivalent 1994 category LR-cd or LR-nt). 2 Potential Proxies – Camels: Camelus dromedaries, C. ferus, Lama guanicoe, Vicugna vicugna; Equids: Equus caballus, E. przewalksi, E. hemionus; Cheetah: Acinonyx jubatus; Lion: Panthera leo; Elephants: Elephas maximus, Loxodonta africana; Bolson Tortoise: Gopherus flavomarginatus 3 Predation on mule deer (Odocoileus hemionus) and elk (Cervus elaphus) would be limited latitudinally by weather; 4 Work in Namibia has demonstrated coexistence with ranchers and cheetah through education and alternative pastoral practices (ref. 70); 5 ref. 8,41,55 6 ref. 8,41,75,76; 7 ref 41,66; 8 ref 54 FIGURE CAPTIONS FIGURE 1. (A) Body size distributions (log body mass) of terrestrial North American mammals (including bats) before (red) and after (grey) late Pleistocene (LP) extinctions. (B) Body size distributions (kg) of four large-bodied taxonomic groups before and after LP extinctions. Extant distribution of Perrisodactyla includes feral horses and burros. FIGURE 2. A qualitative model for the biological, economic, and esthetic components of Pleistocene rewilding. Iconic symbols are for condor, horses, Bolson tortoises, camelids, cheetah, Asian and African elephants, and lions (see Table 1 for Latin names). (a) Area requirements and likely timescale of reintroducing functional analogs of extinct megafauna to North America (b) Conservation value and ecological role (interactivity) on the landscape (c) Potential economic-cultural value versus conflict. FIGURE 1. FIGURE 2.