* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Thyroid hormones
Neuroendocrine tumor wikipedia , lookup
Breast development wikipedia , lookup
Xenoestrogen wikipedia , lookup
Glycemic index wikipedia , lookup
Mammary gland wikipedia , lookup
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency wikipedia , lookup
Hyperandrogenism wikipedia , lookup
Hyperthyroidism wikipedia , lookup
Endocrine disruptor wikipedia , lookup
Graves' disease wikipedia , lookup
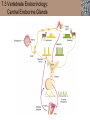
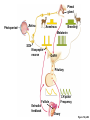
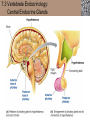
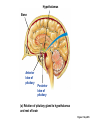
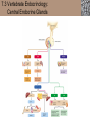
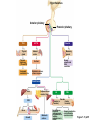
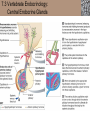
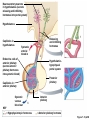
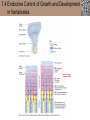
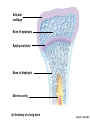
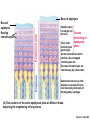
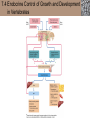
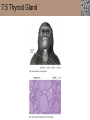
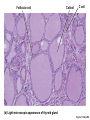
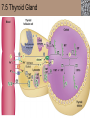
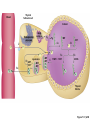
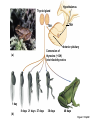
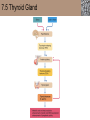
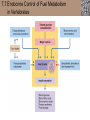
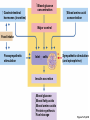
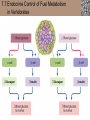
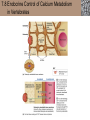
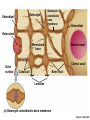
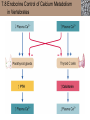
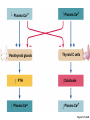
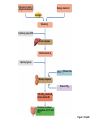
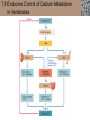
Lauralee Sherwood Hillar Klandorf Paul Yancey Chapter 7 Endocrine Systems Kip McGilliard • Eastern Illinois University 7.1 Introduction: Principles of Endocrinology Endocrinology is the study of the evolution and physiological function of hormones. • The endocrine system regulates and coordinates distant organs through the secretion of hormones. • Hormones are signal molecules delivered by circulatory fluids. • In contrast to the nervous system, the endocrine system controls activities that require duration rather than speed. 7.1 Introduction: Principles of Endocrinology Chemical classes of hormones • Peptide and protein hormones • Chains of amino acids • Hydrophilic • Example: Insulin • Amines • Derived from tyrosine • Catecholamines (e.g. epinephrine) are hydrophilic • Thyroid hormones (e.g. thyroxine) are lipophilic • Steroids • Derived from cholesterol • Lipophilic • Examples: Testosterone and estradiol 7.1 Introduction: Principles of Endocrinology Hormone synthesis and secretion • Peptide hormones • Synthesized as large precursor proteins, preprohormones • Portions are cleaved and peptide hormone is packaged into secretory vesicles • Released from cell by exocytosis • Steroid hormones • Cholesterol is synthesized or obtained from diet • Chemically modified by a series of enzymatic reactions • Once synthesized, steroid hormones immediately diffuse across the plasma membrane 7.1 Introduction: Principles of Endocrinology Cholesterol Pregneneolone 17-Hydroxypregneneolone Progesterone 17-Hydroxyprogesterone Androstenedione Estrone Deoxycortisol Testosterone Estradiol Dehydroepiandrosterone (adrenal cortex hormone) (female sex hormone) 11-Deoxycorticosterone Androgens (male sex hormones) Corticosterone Aldosterone Mineralocorticoid (adrenal cortex hormone) Cortisol Estriol Glucocorticoid (adrenal cortex hormone) Estrogens (female sex hormones) Figure 7-3 p272 7.1 Introduction: Principles of Endocrinology Mechanisms of hormone action • Hormones are widely distributed, but only target cells have receptors to respond to each hormone • Peptides and catecholamines bind with membrane receptors • Alter the conformation of adjacent ion channels, or • Activate second-messenger systems • Steroid and thyroid hormones pass through the plasma membrane and bind with internal receptors • Receptors inside the cell are transcription factors that regulate specific genes • Hormone receptor complex binds with hormone response element (HRE) on nuclear DNA • Turns on synthesis of a specific protein 7.1 Introduction: Principles of Endocrinology Blood vessel Plasma protein carrier Steroid hormone ECF Plasma membrane Cytoplasm Cellular response 1 Free lipophilic hormone 9 New protein brings about desired response. diffuses though plasma membrane Steroid hormone receptor New protein Portion that binds hormone 8 New protein is released from ribosome and processed into final folded form. Portion that binds to DNA 2 Hormone binds with 7 Ribosomes “read” mRNA to synthesize new proteins. intracellular receptor specific for it. DNA-binding site (active) 6 New mRNA leaves nucleus. 3 Hormone receptor complex binds with DNA’s hormone response element. mRNA 4 Binding activates gene. 5 Activated gene transcribes mRNA. DNA Nucleus Hormone Gene response element Figure 7-4 p274 7.1 Introduction: Principles of Endocrinology Regulation of plasma concentration of hormones • Negative feedback control • When plasma hormone levels fall, hormone secretion is stimulated • Neuroendocrine reflexes • Produce a sudden increase in hormone secretion in response to a specific stimulus • Biological rhythms • Secretion of most hormones rhythmically fluctuates as a function of time (biological clocks) • Readjustment of set point by CNS • Example: Cortisol secretion rises at night to peak in early morning (diurnal rhythm) 7.1 Introduction: Principles of Endocrinology 7.1 Introduction: Principles of Endocrinology Endocrine disorders • Hyposecretion -- inadequate secretion of a hormone • Primary hyposecretion -- abnormality within the gland • Secondary hyposecretion -- deficiency of tropic hormones • Hypersecretion -- excessive secretion of a hormone • Primary or secondary • Endocrine-disrupting chemicals (EDCs) • Human-made substances released into the environment that mimic or oppose the actions of hormones • Example: DDE and DDT act as anti-androgens in mammals 7.2 Nonvertebrate Endocrinology Growth and molting in insects • Ecdysone is secreted by the prothoracic glands • Secretion of ecdysone is stimulated by prothoracotropic hormone (PTTH) secreted by neurosecretory cells in brain • Ecdysone initiates the molting process • Juvenile hormone (JH) is secreted by the corpora allata • JH assures that larval characteristics are retained • JH levels progressively decline at each larval stage • Ecdysone in the absence of JH enables metamorphosis to the adult form 7.2 Nonvertebrate Endocrinology Stimuli (related to feeding activities) anterior end of larva Hormone secretory cells in brain Juvenile hormone Brain hormone Prothoracic gland Corpus allatum β α– ecdysone ecdysone 1 2 3 4 Changing blood concentrations of hormones Larval stages Pupa Adult Figure 7-7 p280 7.3 Vertebrate Endocrinology: Central Endocrine Glands Pineal Hypothalamus Pituitary Parathyroid Thyroid Thymus Heart Stomach Adrenal gland Pancreas Duodenum Kidney Skin Ovaries in female Placenta in pregnant female Testes in male Figure 7-1 p269 ANIMATION: Major human endocrine glands To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE 7.3 Vertebrate Endocrinology: Central Endocrine Glands Pineal gland • Secretes melatonin • Maintains circadian rhythms • Melatonin secretion increases up to 10-fold in darkness • Seasonal changes in melatonin secretion patterns trigger reproduction • In mammals melatonin output is controlled by the suprachiasmatic nucleus (SCN) of the hypothalamus • SCN receives light information from the eyes 7.3 Vertebrate Endocrinology: Central Endocrine Glands Pineal gland Photoperiod Retina Anestrous Breeding Melatonin SCN Kisspeptin neuron GnRH Pituitary LH pulse Frequency Follicle Estradiol feedback Ovary Figure 7-8 p282 7.3 Vertebrate Endocrinology: Central Endocrine Glands Pituitary gland (hypophysis) • Located at the base of the brain, connected to the hypothalamus by a thin stalk, the infundibulum • Posterior pituitary (neurohypophysis) • Nervous tissue • Anterior pituitary (adenohypophysis) • Glandular epithelial tissue • Intermediate lobe (pars intermedia) • Absent in birds and cetaceans • Rudimentary in humans after birth • Size of intermediate lobe correlates with ability of animal to adapt to coloration of its environment 7.3 Vertebrate Endocrinology: Central Endocrine Glands Hypothalamus Bone Anterior lobe of pituitary Posterior lobe of pituitary (a) Relation of pituitary gland to hypothalamus and rest of brain Figure 7-9a p285 Hypothalamus Optic chiasm Anterior pituitary Connecting stalk Posterior pituitary (b) Enlargement of pituitary gland and its connection to hypothalamus Figure 7-9b p285 7.3 Vertebrate Endocrinology: Central Endocrine Glands Intermediate lobe • Secretes melanocyte-stimulating hormone (MSH) • α-MSH controls skin coloration via dispersion of storage granules containing melanin • In lower vertebrates, α-MSH is opposed by melaninconcentrating hormone (MCH) • Melanocortin-1 receptor (MC1R) determines skin color, pelage and feather pigmentation in animals lacking pars intermedia • Excessive MSH secretion darkens human skin • MSH reduces appetite and suppresses immune system 7.3 Vertebrate Endocrinology: Central Endocrine Glands Posterior pituitary • Connects to the hypothalamus by a neural pathway • Neurosecretory neurons have cell bodies in supraoptic and paraventricular nuclei of hypothalamus • Axons terminate on capillaries in posterior pituitary • Secretes vasopressin and oxytocin • Evolutionary precursor, arginine vasotocin, is found in many vertebrates 7.3 Vertebrate Endocrinology: Central Endocrine Glands Posterior pituitary hormones • Vasopressin • Enhances retention of water by kidneys (antidiuretic effect) • Causes contraction of arteriolar smooth muscle (vasoconstriction) • Oxytocin • Social bonding • Contraction of uterine smooth muscle • Ejection of milk from mammary glands • Arginine vasotocin • Involved in osmoregulation • Vasoconstriction 7.3 Vertebrate Endocrinology: Central Endocrine Glands Supraoptic nucleus 1 Neurosecretory neuronal cell bodies in hypothalamus (produce vasopressin and oxytocin) Hypothalamus Paraventricular nucleus Axons Hypothalamic posteriorpituitary stalk Capillary Anterior pituitary Posterior pituitary Systemic arterial blood in Vasopressin Neuronal terminals in posterior pituitary (release vasopressin and oxytocin into systemic blood) Systemic venous blood out Oxytocin Figure 7-10 p286 Vasopressin Nephrons in kidneys Increases permeability of distal and collecting tubules to H2O Arterioles throughout body Causes vasoconstriction Oxytocin Uterus Stimulates uterine contractions Mammary glands Stimulates milk ejection during breastfeeding Figure 7-10 p286 7.3 Vertebrate Endocrinology: Central Endocrine Glands Anterior pituitary hormones • Growth hormone (GH, somatotropin) • Stimulates growth and affects metabolism • Thyroid-stimulating hormone (TSH, thyrotropin) • Stimulates thyroid hormone secretion by thyroid gland • Adrenocorticotropic hormone (ACTH, corticotropin) • Stimulates cortisol secretion by the adrenal cortex • Follicle-stimulating hormone (FSH) • Regulates gamete production • Luteinizing hormone (LH) • Regulates sex hormone secretion • Ovulation and formation of corpus luteum in females • Prolactin (PRL) • Stimulates milk production by mammary glands • Wide range of additional actions 7.3 Vertebrate Endocrinology: Central Endocrine Glands Hypothalamus Anterior pituitary Posterior pituitary TSH ACTH Thyroid gland Prolactin Adrenal cortex Thyroid hormone (T3 and T4) Mammary glands Breast growth and milk secretion Cortisol Increased metabolic rate Metabolic actions; stress response Growth hormone Adipose tissue, muscle, liver Liver IGF-I Bone (ovaries in females) Soft tissues Growth LH Metabolic actions FSH Gonads Sex hormone secretion (estrogen and progesterone in females, testosterone in (testes in males) Gamete production (ova in females, sperm in males) Figure 7-11 p287 ANIMATION: Anterior pituitary function To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE 7.3 Vertebrate Endocrinology: Central Endocrine Glands Hypothalamic releasing and inhibiting hormones • Secretion of each anterior pituitary hormone is regulated by hypothalamic hypophysiotropic hormones • Thyrotropin-releasing hormone (TRH) • Corticotropin-releasing hormone (CRH) • Gonadotropin-releasing hormone (GnRH) stimulates release of FSH and LH • Growth hormone-releasing hormone (GHRH) • Growth hormone-inhibiting hormone (GHIH, somatostatin) • Prolactin-releasing hormone (PRH) • Prolactin-inhibiting hormone (PIH) 7.3 Vertebrate Endocrinology: Central Endocrine Glands Hypothalamic releasing and inhibiting hormones • Releasing and inhibiting hormones reach the anterior pituitary through the hypothalamic-hypophyseal portal system • Regulation of hypophysiotropic hormone secretion • Neural input (e.g. CRH secretion in response to stress) • Negative-feedback effects of anterior pituitary or target gland hormones (e.g. cortisol levels above a set point inhibit CRH and ACTH secretion) 7.3 Vertebrate Endocrinology: Central Endocrine Glands Neurosecretory neurons in hypothalamus (secrete releasing and inhibiting hormones into portal system) Hypothalamus 1 Capillaries in hypothalamus Systemic arterial blood in Endocrine cells of anterior pituitary (secrete anterior pituitary hormones into systemic blood) 1 2 Hypothalamichypophyseal portal system 3 Posterior pituitary 4 Capillaries in anterior pituitary Systemic venous blood out Releasing and inhibiting hormones 5 6 Anterior pituitary KEY = Hypophysiotropic hormones = Anterior pituitary hormone Figure 7-13 p289 7.3 Vertebrate Endocrinology: Central Endocrine Glands 7.4 Endocrine Control of Growth and Development in Vertebrates Growth depends on: • Adequate diet • Malnourished animals do not reach full growth potential • Seasonally shortened day length reduces growth by reducing food intake • Freedom from chronic disease and stressful environmental conditions • Glucocorticoids secreted during stress inhibit growth • Growth-influencing hormones • Placental hormones promote fetal growth • Growth hormone and other hormones promote growth after birth 7.4 Endocrine Control of Growth and Development in Vertebrates Direct effects of growth hormone (GH) • Metabolic effects • Target organs are adipose tissue, skeletal muscles and liver • Mobilizes fat stores as a major energy source • Conserves glucose for use by the brain • Decreases glucose uptake by muscles and increases glucose output by the liver • Enhances immune system GH’s growth-promoting actions are mediated by insulin-like growth factors (IGFs) 7.4 Endocrine Control of Growth and Development in Vertebrates GH/IGF’s growth promoting effects • Growth of soft tissues • • • • Increases number of cells (hyperplasia) Increases size of cells (hypertrophy) Promotes uptake of amino acids into cells Stimulates protein synthesis and inhibits protein degradation • Growth of bone • Promotes increases in bone thickness and length • Thickness depends on addition of new bone by osteoblasts • Length depends on proliferation of cartilage cells (chondrocytes) in epiphyseal plates and invasion by osteoblasts 7.4 Endocrine Control of Growth and Development in Vertebrates Articular cartilage Bone of epiphysis Epiphyseal plate Bone of diaphysis Marrow cavity (a) Anatomy of a long bone Figure 7-14a p294 Bone of epiphysis Diaphysis Resting chondrocytes Epiphyseal plate Bone of epiphysis Chondrocytes 1 undergo cell Causes division. thickening of epiphyseal The 2 older chondrocytes plate grow larger. As the extracellular matrix calcifies, the entrapped chondrocytes die. The dead chondrocytes are cleared away by osteoclasts. Osteoblasts swarm up from diaphysis and deposit bone over persisting remnants of disintegrating cartilage. (b) Two sections of the same epiphyseal plate at different times, depicting the lengthening of long bones Figure 7-14b p294 7.4 Endocrine Control of Growth and Development in Vertebrates Regulation of growth hormone secretion • Negative feedback loop involving hypothalamuspituitary-liver axis • IGF-I inhibits secretion of GH by somatotropes in anterior pituitary • IGF-I inhibits GHRH-secreting cells and stimulates somatostatin-secreting cells in hypothalamus • Other stimuli to GH secretion • • • • Onset of sleep Exercise, stress, and hypoglycemia High protein meal Ghrelin 7.4 Endocrine Control of Growth and Development in Vertebrates Bone of epiphysis Diaphysis Resting chondrocytes Epiphyseal plate Bone of epiphysis Chondrocytes 1 undergo cell Causes division. thickening of epiphyseal The 2 older chondrocytes plate grow larger. As the extracellular matrix calcifies, the entrapped chondrocytes die. The dead chondrocytes are cleared away by osteoclasts. Osteoblasts swarm up from diaphysis and deposit bone over persisting remnants of disintegrating cartilage. (b) Two sections of the same epiphyseal plate at different times, depicting the lengthening of long bones Figure 7-14b p294 7.4 Endocrine Control of Growth and Development in Vertebrates Growth hormone administration • Increases bone growth • Treatment of dwarfism in humans • Increases muscle mass • Abuse by athletes • Improved meat production in swine • Increases milk production in dairy cattle 7.5 Thyroid Gland Thyroid gland is located in the throat below the larynx • Composed of follicular cells arranged in fluidfilled spheres (thyroid follicles) • Colloid serves as an extracellular storage site for thyroid hormones in the form of thyroglobulin, a large glycoprotein 7.5 Thyroid Gland Thyroid gland Right lobe Trachea Isthmus Left lobe (a) Gross anatomy of thyroid gland Figure 7-16a p298 Follicular cell Colloid C cell (b) Light-microscopic appearance of thyroid gland Figure 7-16b p298 7.5 Thyroid Gland Thyroid hormone synthesis 1. Thyroglobulin (Tg) is synthesized by thyroid follicular cells (incorporating tyrosine) and secreted into colloid by exocytosis 2. Thyroid follicular cells efficiently capture iodide (I-), obtained from the diet, using an iodide pump 3. Iodide is activated and attached to tyrosine molecules on Tg in colloid • • Monoiodotyrosine (MIT) has one iodine Diiodotyrosine (DIT) has two iodines 4. Iodinated tyrosines couple to form tetraiodothyronine (T4, thyroxine) and triiodothyronine (T3) 7.5 Thyroid Gland Secretion of thyroid hormones 1. Follicular cells take up a piece of colloid (containing iodinated Tg) by phagocytosis 2. Lysosomal enzymes split off T4, T3, MIT and DIT in the process of breaking down Tg 3. T4 and T3 (biologically active thyroid hormones) diffuse across follicular cell membrane into blood, while MIT and DIT are recycled to iodide and tyrosine 7.5 Thyroid Gland Thyroid follicular cell Blood Colloid Endoplasmic reticulum 2 I– Golgi complex Tg MIT Lysosome DIT 7 T3 T4 MIT DIT T3 T4 4b I– 3 MIT DIT T3 T4 DIT I– I– 8b MIT 8a Tg 4a I– T3 T4 1 5a 6 5b 2 DITs 1 MIT + 1 DIT T3 T4 Thyroid follicle Figure 7-17 p299 7.5 Thyroid Gland Mechanism of thyroid hormone action • T3 is the major biologically active form of thyroid hormone • Most secreted T4 is activated by conversion to T3 by a deiodinase enzyme • T3 binds with nuclear receptors attached to thyroid-response elements of DNA • Alters transcription of specific mRNAs and synthesis of specific proteins 7.5 Thyroid Gland Effects of thyroid hormones • Increase basal metabolic rate (BMR) through increased mitochondrial and Na+-K+ pump activity • Modulate synthesis and degradation of metabolic fuel molecules • Molting in birds and mammals • Sympathomimetic effect -- increase target cell • • • • responsiveness to catecholamines Increase heart rate and force of contraction Essential for growth (permissive effect on GH) Development of CNS Metamorphosis in amphibians 7.5 Thyroid Gland Hypothalamus Thyroid gland TRH TSH Anterior pituitary Conversion of thyroxine (+ GH) into triiodothyronine (a) 1 day 8 days 21 days 27 days 30 days 40 days (b) Figure 7-18 p302 7.5 Thyroid Gland Regulation of thyroid hormone secretion • Negative feedback loop involving hypothalamus-pituitary-thyroid axis • Thyroid-stimulating hormone (TSH) stimulates almost every step of thyroid hormone synthesis and secretion • Hypothalamic thyrotropin-releasing hormone (TRH) stimulates TSH secretion by thyrotropes in anterior pituitary • Elevated T3 and T4 levels inhibit TSH secretion • Other factors affecting thyroid hormone secretion • Stress inhibits TSH secretion • Cold stimulates TSH secretion (infants) 7.5 Thyroid Gland Stress Cold in infants Hypothalamus Thyrotropin-releasing hormone (TRH) Anterior pituitary Thyroid-stimulating hormone (TSH) Thyroid gland Thyroid hormone (T3 and T4) Metabolic rate and heat production; enhancement of growth and CNS development; enhancement of sympathetic activity Figure 7-19 p303 7.5 Thyroid Gland Abnormalities of thyroid function • Hypothyroidism -- low thyroid activity • Causes • Primary failure of thyroid gland or • Secondary to a deficiency of TSH (or TRH) or • Inadequate dietary supply of iodine • Symptoms stem from reduced metabolic activity (e.g. weight gain, fatigue, poor tolerance of cold) • Hyperthyroidism -- elevated thyroid activity • Symptoms stem from increased metabolic activity (e.g. weight loss, increased heart rate, anxiety) 7.6 Adrenal Glands Adrenal glands are located above the kidneys • Outer adrenal cortex is composed of steroidogenic cells of mesodermal origin • Inner adrenal medulla is composed of chromaffin cells of neural crest origin • Steroidogenic and chromaffin tissues are intermingled in most non-mammalian species 7.6 Adrenal Glands Steroid hormones of the adrenal cortex • Derived from cholesterol • Modified by stepwise enzymatic reactions • Mineralocorticoids (e.g. aldosterone) • Influence mineral (electrolyte) balance • Produced in zona glomerulosa • Glucocorticoids (e.g. cortisol) • Role in metabolism of glucose, proteins and lipids • Produced in zona fasciculata • Sex steroids (e.g. dehydroepiandrosterone) • Androgenic (masculinizing) effects • Produced in zona fasciculata and zona reticularis 7.6 Adrenal Glands Adrenal cortex Adrenal medulla Adrenal gland Kidney (a) Location and gross structure of adrenal glands Figure 7-20a p305 Mineralcorticoids Connective tissue capsule Zona glomerulosa Zona fasciculata Cortex Glucocorticoids (sex hormones) Zona reticularis Medulla Catecholamines (b) Layers of adrenal cortex Figure 7-20b p305 7.6 Adrenal Glands Cholesterol Pregneneolone 17-Hydroxypregneneolone Progesterone 17-Hydroxyprogesterone Androstenedione Estrone Deoxycortisol Testosterone Estradiol Dehydroepiandrosterone (adrenal cortex hormone) (female sex hormone) 11-Deoxycorticosterone Androgens (male sex hormones) Corticosterone Aldosterone Mineralocorticoid (adrenal cortex hormone) Cortisol Estriol Glucocorticoid (adrenal cortex hormone) Estrogens (female sex hormones) Figure 7-3 p272 7.6 Adrenal Glands Effects of glucocorticoids • Metabolic effects -- increase blood glucose, while reducing protein and fat stores • Permissive actions (e.g. permit catecholamines to induce vasoconstriction) • Enhanced memory • Adaptation to long-term stress • Anti-inflammatory and immunosuppressive effects, especially at high doses 7.6 Adrenal Glands Regulation of glucocorticoid secretion • Negative feedback loop involving hypothalamus-pituitary-adrenal axis • Adrenocorticotropic hormone (ACTH) stimulates cortisol secretion • Hypothalamic corticotropin-releasing hormone (CRH) stimulates ACTH secretion by corticotropes in the anterior pituitary • Elevated glucocorticoid levels inhibit CRH and ACTH secretion • Other factors affecting glucocorticoid secretion • Stress stimulates CRH secretion • Circadian rhythm 7.6 Adrenal Glands Stress Diurnal rhythm Hypothalamus Corticotropin-releasing hormone (CRH) Anterior pituitary Adrenocorticotropic hormone (ACTH) Adrenal cortex Cortisol Metabolic fuels and building blocks available to help resist stress Blood glucose (by stimulating gluconeogenesis and inhibiting glucose uptake) Blood amino acids (by stimulating protein degradation) Blood fatty acids (by stimulating lipolysis) Figure 7-21 p307 7.6 Adrenal Glands Abnormalities of adrenocortical function • Cushing’s syndrome -- excessive cortisol secretion • Most common cause -- overstimulation by excess ACTH • Consequences of excessive gluconeogenesis • High blood glucose and protein loss • Redistribution of fat in humans and dogs • Addison’s disease (primary adrenocortical insufficiency) -- deficiency of adrenal steroids • Most common cause -- autoimmune destruction of the adrenal cortex • Aldosterone deficiency can be fatal • Cortisol deficiency causes poor response to stress, hypoglycemia, and lack of permissive actions 7.6 Adrenal Glands Chromaffin cells in the adrenal medulla are modified postganglionic sympathetic neurons. • Secrete norepinephrine (NE) and epinephrine (ratio varies between species) • Both are catecholamines derived from tyrosine • Most synthetic steps take place in cytoplasm • Stored in chromaffin granules • Secretion is by exocytosis (similar to neurotransmitter secretion) • Secretion is stimulated by the sympathetic system (e.g. during fear or stress) 7.6 Adrenal Glands Effects of adrenal catecholamines • Increased cardiac output and arterial blood pressure • Vasodilation of coronary and skeletal-muscle arterioles • Dilation of respiratory airways • Inhibition of digestive activity • Mobilization of stored carbohydrates and fat • CNS arousal • Sweating • Dilation of pupils and flattening of lens 7.6 Adrenal Glands Multifaceted stress response is coordinated by the hypothalamus • Hypothalamus receives input concerning physical and emotional stressors • Activates sympathetic nervous system • Secretes CRH • Secretes vasopressin • Chronic stress responses are detrimental • Breakdown of body structures • Reproductive failure • Increased susceptibility to disease 7.6 Adrenal Glands 7.7 Endocrine Control of Fuel Metabolism in Vertebrates Metabolism refers to all chemical reactions that occur within body cells. • Anabolism -- synthesis of larger organic molecules from smaller subunits • Requires energy in the form of ATP • Manufacture of molecules needed by the cell • Storage of nutrients • Catabolism -- breakdown of organic molecules into smaller subunits • Hydrolysis of large organic macromolecules • Oxidation of smaller molecules (e.g. glucose) to release energy for ATP production 7.7 Endocrine Control of Fuel Metabolism in Vertebrates 7.7 Endocrine Control of Fuel Metabolism in Vertebrates Regulation of metabolic fuels • Dietary intake is usually intermittent • Absorptive state • After a meal • Glucose is plentiful and used as the major energy source • Excess nutrients are stored as glycogen or triglycerides • Postabsorptive state • Between meals (fasting) • Endogenous energy stores are mobilized to provide energy • Fatty acids are the major energy source for most tissues 7.7 Endocrine Control of Fuel Metabolism in Vertebrates Pancreas is composed of both exocrine and endocrine tissues • Exocrine portion secretes digestive enzymes through the pancreatic duct into the digestive tract lumen • Islets of Langerhans are integrators of endocrine regulatory responses and secrete hormones • Pancreatic hormones are the dominant hormonal regulators of glucose homeostasis • • • • β cells secrete insulin α cells secrete glucagon D cells secrete somatostatin F cells secrete pancreatic polypeptide 7.7 Endocrine Control of Fuel Metabolism in Vertebrates Effects of insulin • Lowers blood glucose and promotes storage of carbohydrates • • • • Facilitates glucose transport into most cells Stimulates glycogenesis in skeletal muscle and liver Inhibits glycogenolysis in liver Inhibits gluconeogenesis in liver • Lowers blood fatty acids and promotes storage of triglycerides • Stimulates production of fatty acids from glucose • Inhibits lipolysis • Lowers blood amino acids and enhances protein synthesis • Promotes uptake of amino acids into cells 7.7 Endocrine Control of Fuel Metabolism in Vertebrates Factors that increase blood glucose Factors that decrease blood glucose Transport of glucose into cells: ––For utilization for energy production ––For storage *as glycogen through glycogenesis *as triglycerides Glucose absorption from digestive tract Blood glucose Hepatic glucose production: ––Through glycogenolysis of stored glycogen ––Through gluconeogenesis Urinary excretion of glucose (occurs only abnormally, when blood glucose level becomes so high it exceeds the reabsorptive capacity of kidney tubules during urine formation) Figure 7-24 p317 ANIMATION: Hormones and glucose metabolism To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE 7.7 Endocrine Control of Fuel Metabolism in Vertebrates Regulation of insulin secretion • Direct negative-feedback system between pancreatic β cells and the blood glucose level • During absorption of a meal, insulin secretion increases • Other factors that stimulate insulin secretion: • Increased blood amino acids • Gastrointestinal hormones -- glucoseindependent insulinotropic peptide (GIP), glucagon-like peptide (GLP) • Increased parasympathetic activity 7.7 Endocrine Control of Fuel Metabolism in Vertebrates Gastrointestinal hormones (incretins) Blood glucose concentration Blood amino acid concentration Major control Food intake Parasympathetic stimulation Islet cells Sympathetic stimulation (and epinephrine) Insulin secretion Blood glucose Blood fatty acids Blood amino acids Protein synthesis Fuel storage Figure 7-25 p319 7.7 Endocrine Control of Fuel Metabolism in Vertebrates Glucagon • Effects oppose those of insulin • Increases hepatic glucose production and raises blood glucose levels • Promotes fat breakdown and inhibits triglyceride synthesis, raising fatty acid levels in blood • Promotes protein breakdown in liver, but does not affect muscle protein • Glucagon secretion is increased during the postabsorptive state when blood glucose levels are low 7.7 Endocrine Control of Fuel Metabolism in Vertebrates Blood glucose β cell α cell Glucagon Insulin Blood glucose to normal Blood glucose α cell β cell Glucagon Insulin Blood glucose to normal Figure 7-26 p321 Blood glucose Blood glucose a cell b cell a cell b cell Glucagon Insulin Glucagon Insulin Blood glucose to normal Blood glucose to normal Stepped Art Figure 7-26 p321 7.7 Endocrine Control of Fuel Metabolism in Vertebrates Diabetes mellitus • Elevated blood glucose levels (hyperglycemia) • Glucose in the urine attracts water to cause excessive urination • Type I (insulin-dependent) diabetes mellitus • Lack of insulin secretion by pancreatic β cells • Requires administration of insulin • Type II (non-insulin-dependent) diabetes mellitus • Insulin levels are normal or elevated • Reduced sensitivity of target cells to insulin 7.8 Endocrine Control of Calcium Metabolism in Vertebrates Importance of calcium • In mammals, 99% of calcium (Ca2+) is stored in the skeleton and teeth • Only free Ca2+ in plasma is biologically active and subject to regulation • Both Ca2+ homeostasis and Ca2+ balance must be regulated • Ca2+ plays a vital role in: • Neuromuscular excitability • Excitation-contraction coupling in cardiac and smooth muscle • Stimulus-secretion coupling • Maintenance of tight junctions between cells • Clotting of blood 7.8 Endocrine Control of Calcium Metabolism in Vertebrates Parathyroid hormone (PTH) • Secreted by the parathyroid glands, located near the thyroid gland • Essential for life • Raises plasma Ca2+ levels • • • • Promotes transfer of Ca2+ from bone fluid into plasma Promotes resorption of bone by osteoclasts Increases reabsorption of Ca2+ in the kidneys Indirectly increases Ca2+ absorption from the small intestine by activating vitamin D • PTH secretion is increased in response to a fall in plasma Ca2+ levels 7.8 Endocrine Control of Calcium Metabolism in Vertebrates Osteoblast Osteocyte Osteocytic– osteoblastic bone membrane Osteoblast Osteoclast Mineralized bone Outer surface Blood vessel Central canal Canaliculi Bone fluid Lamellae (a) Osteocytic–osteoblastic bone membrane Figure 7-30a p328 In canaliculi Mineralized bone: stable pool of Ca2+ In central canal Bone fluid: labile pool of Ca2+ Fast exchange 1 2 Slow exchange (Bone dissolution) Plasma Ca2+ Ca2+ Osteocytic–osteoblastic bone membrane (formed by filmy cytoplasmic extensions of interconnected osteocytes and osteoblasts) (b) Fast and slow exchange of Ca2+ between bone and plasma Figure 7-30b p328 7.8 Endocrine Control of Calcium Metabolism in Vertebrates Plasma Ca2+ Plasma Ca2+ Parathyroid glands Thyroid C cells PTH Plasma Ca2+ Calcitonin Plasma Ca2+ Figure 7-31 p328 7.8 Endocrine Control of Calcium Metabolism in Vertebrates Calcitonin • Produced by C cells of the mammalian thyroid gland, ultimobranchial glands in birds, and connective tissue sheets around the heart in fishes • Decreases plasma Ca2+ levels • Decreases transfer of Ca2+ from bone fluid into plasma • Decreases bone resorption by inhibiting activity of osteoclasts • Ability to lower blood Ca2+ is especially important in marine fishes because of Ca2+ in sea water • Calcitonin secretion is increased in response to an increase in plasma Ca2+ levels 7.8 Endocrine Control of Calcium Metabolism in Vertebrates Vitamin D (cholecalciferol) • Produced in skin from 7-dehydrocholesterol on exposure to sunlight • Can also be obtained in the diet • Activated by sequential alterations in the liver and kidneys, forming 1,25-(OH)2-vitamin D3 (calcitriol) • Promotes Ca2+ absorption in the intestine • Increases sensitivity of bone to PTH 7.8 Endocrine Control of Calcium Metabolism in Vertebrates Precursor in skin (7dehydrocholesterol) Dietary vitamin D Sunlight Vitamin D3 Hydroxyl group (OH) Liver enzymes 25-OH-vitamin D3 Hydroxyl group PTH Plasma Ca2+ Kidney enzymes Plasma PO43 − 1,25-(OH)2 -vitamin D3 (active vitamin D) Promotes intestinal absorption of Ca2+ and PO43 − Figure 7-32 p329 7.8 Endocrine Control of Calcium Metabolism in Vertebrates Relieves Plasma Ca2+ Parathyroid glands PTH Kidneys Enhances responsiveness of bone to PTH Renal tubular Ca2+ reabsorption Activation of vitamin D Bone Mobilization of Ca2+ from bone Intestine Urinary excretion of Ca2+ Absorption of Ca2+ in intestine Plasma Ca2+ Figure 7-33 p330 7.8 Endocrine Control of Calcium Metabolism in Vertebrates Disorders of Ca2+ metabolism • Hyperparathyroidism -- excess PTH secretion • “Bones, stones, and abdominal groans” • Vitamin D deficiency • Impaired intestinal absorption of Ca2+ • Demineralized bone is soft and deformed • Rickets in children; osteomalacia in adults • Excessive demands for Ca2+ • Parturient paresis (milk fever) in dairy cattle • Egg laying in birds