* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Quantum Magnetic Dipoles and Angular Momenta in SI Units
Copenhagen interpretation wikipedia , lookup
Coherent states wikipedia , lookup
History of quantum field theory wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
EPR paradox wikipedia , lookup
Renormalization group wikipedia , lookup
Hidden variable theory wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Quantum state wikipedia , lookup
Canonical quantization wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Magnetoreception wikipedia , lookup
Electron configuration wikipedia , lookup
Atomic orbital wikipedia , lookup
Spin (physics) wikipedia , lookup
Atomic theory wikipedia , lookup
Electron scattering wikipedia , lookup
Magnetic monopole wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
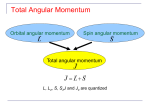
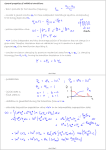
Quantum Magnetic Dipoles and Angular Momenta in SI Units Bohr Magneton It seems to be agreed upon that in SI units, we define the Bohr magneton µB and the nuclear magneton µp by e~ µB ≡ 2m e~ µp ≡ 2mp where e is the proton charge (and the negative of the electron charge), m is the electron mass, and mp is the proton mass (or also possibly the neutron mass, according to Abers’ [1] notation). So a “magneton” has the same dimension as magnetic dipole moment (µ). See, for example, [ http://physics.nist.gov/cgi-bin/cuu/Value?eqmun ] for verification. Relation of Magnetic Dipole Moment to the Magneton We derive the electron’s orbital magnetic dipole moment µ and find the motivation for the definition of the Bohr magneton. Using the assumptions of Bohr’s atomic model, the electron circulates in a flat orbit of radius r, with angular momentum L = |r × p| = mrv, and evr v e e (πr2 ) = − µ = IA = −e =− mvr = − L 2πr 2 2m 2m p Since we may have L = ~ l(l + 1), this becomes p e~ p l(l + 1) ≡ µB l(l + 1) µ=− 2m so we see how the orbital magnetic dipole moment of the electron is quantized in terms of µB . (So a magneton is a quantum of magnetic dipole moment.) Note that when we include spin, we get e e µB µB µ=− L−g S =− L−g S (1) 2m 2m ~ ~ Angular Momentum Operator Definitions and Conventions Now, in quantum mechanics we have the freedom to define the angular momentum operators (Ĵ, L̂, and Ŝ) and the magnetic dipole moment operator (µ̂) using different conventions: we may define them to have the same dimension as angular momentum and magnetic dipole moment, respectively, or we may define them to be dimensionless. Taking the operator Ĵ for example, we may have p Ĵ ≡ r̂ × p̂ so that J = ~ j(j + 1) when it acts on angular momentum eigenstates, or we may have p ~Ĵ ≡ r̂ × p̂ so that J = j(j + 1) when it acts on angular momentum eigenstates. The table below summarizes these options and the resulting consequences for equation 1 above. Possible Conventions Resulting Equation Ĵ, L̂, Ŝ “dimensionful” µ̂ “dimensionful” µ̂ = − µ~B L̂ − g µ~B Ŝ Ĵ, L̂, Ŝ “dimensionful” µ̂ dimensionless µ̂ = − ~1 L̂ − g ~1 Ŝ Ĵ, L̂, Ŝ dimensionless µ̂ “dimensionful” µ̂ = −µB L̂ − gµB Ŝ Ĵ, L̂, Ŝ dimensionless µ̂ dimensionless µ̂ = −L̂ − g Ŝ 1 References [1] Ernest S. Abers: Quantum Mechanics, Pearson, Prentice Hall (2004) 2