* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Regional Oxygen Saturation of Small Arteries and Veins in the
Heart failure wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
119
Regional Oxygen Saturation of Small Arteries and
Veins in the Canine Myocardium
HARVEY R. WEISS AND A.K.
SINHA
Downloaded from http://circres.ahajournals.org/ by guest on June 14, 2017
SUMMARY Oxygen saturation of small arteries and veins (20-500 fim) was determined microspectrophotometrically in the hearts of 12 pentobarbital-anesthetized open-chest dogs. Hearts were removed, quick frozen in liquid
propane, and O2 saturation was determined in blood vessels on a regional basis between and within ventricular
walls. No significant differences existed in arterial O2 saturation between right, left, and septal walls or regionally
within any wall by depth or in base-to-apex comparisons. Although there was variation in arterial saturation, it was
independent of vessel size. Arteries were followed by serial section into the left ventricular wall for distances up to
7.5 mm without significant saturation change. The average venous saturations of the right, septal, and left
ventricular walls were not significantly different. No regional differences in venous saturation were found within any
ventricular wall in comparisons between base and apex. In the left ventricle, subepicardial venous saturation
(29.8%) was significantly higher than subendocardial saturation (16.4%). In veins traced from the surface,
saturation decreased with depth. Greater variability of saturation was found in small compared to large veins. The
greater O2 extraction in the subendocardium may indicate a higher O2 consumption than in the subepicardium.
THE HEART usually extracts about two thirds of the O2
presented to it under normal conditions. There are differences in work load between right, septal, and left ventricular walls and differences in blood flow between ventricular walls.1 Differences in arterial-venous O2 extraction
between ventricular walls have not been determined and
are, in part, the reason for the present study.
Evidence exists that, in the left ventricular free wall,
the deeper, subendocardial (ENDO) region is less dependent on aerobic metabolism than the more superficial,
subepicardial (EPI) region. This difference has been
shown by polarography,2-:l by mass specrroscopy,4 by
measurements of enzyme activity levels,5'" and in isolated
hearts by microscopic oximetry of veins.7 If such differences do, in fact, exist, the ENDO could be less dependent
on aerobic metabolism than the EPI for several reasons:
diffusional loss of oxygen could occur in arteries before
they reach the ENDO; there is a countercurrent arrangement of vessels which shunts O2 away from the ENDO;
the ENDO has a higher oxygen consumption; the ENDO
has the same oxygen consumption as the EPI but a lower
blood flow and, hence, a greater O2 extraction.
It has been reported that vascular and perivascular O2
tension and the O2 saturation of blood decrease with
decreasing arterial vessel diameter in the hamster cheek
pouch.8-9 In the heart, blood supply is from the surface
inward and vessel diameter decreases with depth in the
left ventricular free wall.10 Most arteries on the surface of
the dog heart are associated with two veins in a triad.
Such an arrangement often persists within the wall of the
left ventricle7 and leads to the speculation that a countercurrent exchange of gases could occur within the left
From the Department of Physiology, College of Medicine and Dentistry
of New Jersey, Rutgers Medical School, Piscataway, New Jersey.
Supported by U.S. Public Health Service Grant HL16134 and a Grantin-Aid from the American Heart Association, New Jersey Affiliate.
Address for reprints: Dr. Harvey R. Weiss, Department of Physiology,
CMDNJ-Rutgers Medical School, Piscataway, New Jersey 08854.
Received July 21, 1976; accepted for publication July 22, 1977.
ventricle. There is evidence that the ENDO has a greater
O2 consumption than the EPI." Further, there are reports
that blood flow may be lower in the ENDO.2 To begin to
distinguish between these possible reasons for the differences in the relation between O2 supply and demand in
the left ventricular free wall, regional arterial-venous O2
saturation differences were studied.
We recently developed a microspectrophotometric
method of measurement of O2 saturation of blood with a
high accuracy and repeatability.12- '•' The system allows
accurate determination of the O2 saturation of arteries
and veins in a quick-frozen heart. We have applied it to
measure arterial O2 and venous O2 saturation in the right
and left ventricular free and septal walls and to study
variability of saturation in relation to vessel size.
Methods
Twelve mongrel dogs of either sex, weighing 13.6-28.2
kg, were anesthetized with sodium pentobarbital (30 mg/
kg, iv). Artificial respiration was instituted and the fractional concentration of CO2 in the alveoli (FACO2) w a s
measured with a Godart capnograph and maintained
constant by adjustment of the respirator. One carotid
artery was catheterized. The chest then was opened at the
5th interspace. The pericardium was incised and a partial
cradle was made. In three dogs, the coronary sinus was
also catheterized.
At least one-half hour was allowed for the preparation
to stabilize. Heart rate and blood pressure then were
measured. Arterial blood samples and in three cases
coronary sinus blood samples then were obtained for
analysis of blood gases and pH (IL 113, Instrumentation
Laboratories). Blood O2 saturation (HbO2) was also measured by the method of Van Slyke14 or by a CO-oximeter
(Instrumentation Laboratories).
The heart then was fibrillated to arrest blood flow
during the freezing process. The ventricles were cut with
a large pair of shears below the atrioventricular ring. The
120
CIRCULATION RESEARCH
Downloaded from http://circres.ahajournals.org/ by guest on June 14, 2017
absence of valves allowed rapid filling of the insides of
the ventricles, when they were placed into liquid nitrogencooled liquid propane. Under these conditions, freezing
began simultaneously inside and outside the ventricular
walls. The time from the beginning of fibrillation until the
heart was dropped into the propane averaged 4 seconds.
The frozen hearts were stored at -70°C until analyzed.
To determine the time course of freezing, an equation
for freezing time in skeletal and cardiac muscle tissue
under these conditions was developed. A thermocouple
with a time constant of 1.4 seconds was placed at various
depths in cardiac and skeletal muscle at 37°C, freshly
excised from a dog. The tissues were placed in liquid N2cooled propane and the time required to reach 0°C was
determined. No time differences were observed in cardiac
and skeletal muscle sections of equal thickness, so the
following single equation was developed: freezing time
(sec) = 0.21 [distance (mm)]184. Thus, it should take less
than 8 seconds to freeze to the center of a left ventricular
free wall that was 15 mm thick.
To determine the effect of elapsed time on changes in
arterial and venous O2 saturation, measurements of saturation were performed in two gracilis muscles and the
heart of one additional dog. The muscles were removed
after clamping the blood supply and sections were frozen
at 0 time, and at 15, 30, 60, and 120 seconds. A similar
protocol was followed in the heart. No decrease in arterial
O2 saturation was observed over the 2-minute period
(Fig. 1). No decline in venous O2 saturation was observed
for 15-30 seconds. No difference was seen between veins
<50 fim and larger vessels until 60 seconds elapsed,
when small vein O2 saturation was lower than large.
Hearts were cut on a band saw with an approximately
200-cm-long blade at -20°C. Eight plugs were obtained:
right ventricular base and apex, septal base and apex, and
two left ventricular base and apex plugs. These plugs
were transferred, one by one, into a microtome-cryostat
maintained at —20°C. The tissue sections then were
mounted on a microtome specimen holder and coated
with embedding medium for frozen tissue specimens.
Thirty-/um-thick sections of the plugs were cut on a
rotary microtome and transferred to precooled glass
slides. They were covered with degassed silicone oil and a
cover glass. The slides were transferred to a Zeiss microspectrophotometer, fitted with a cold stage maintained at
— 20°C. No readings were obtained at the edge of any
section.
Two regions of every piece of right ventricle (subepicardial and subendocardial) were examined. Three regions
per plug of septal wall (right, middle, and left) and left
ventricle (subepicardial, middle, and subendocardial) also
were examined. Measurements were made on the first
three to five arteries and veins found in a region, as to
size and O2 saturation. In three hearts, 22 arteries and
veins were followed in serial sections for distances up to
7.5 mm, and measurements were made of diameter and
O2 saturation in each.
O2 saturation of the blood frozen in the myocardial
blood vessels was determined from the ratio of optical
densities at three wavelengths (560, 523, and 506 nm).
This method corrects for light scattering in the frozen
VOL. 42, No. 1, JANUARY
0
15
30
60
Time (Sec)
1978
120
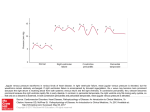
FIGURE 1 The effect of elapsed time before freezing on vessel O 2
saturation. Each point represents a mean of 10 vessels from a
tissue section frozen at successively later times after removal. It
took 18.4, 14.4, and 4.2 seconds for muscle I, muscle 11 (both
gracilis) and heart, respectively, between clamping and putting the
first sample (listed as 0 second) in the liquid propane. The lower
three lines represent the venous O2 saturations from the muscles
and heart.
blood. An average of three readings for each vessel
studied was obtained. The method has been found to
have an accuracy of better than 3% in blood vessels of a
quick-frozen dog gracilis muscle. Details of the accuracy,
precision, and limitations of the method have been previously published.12'13 Inner diameters of each blood vessel,
in which O2 saturation was measured, were determined
with a previously calibrated ocular micrometer.
Figure 2 shows the gross and microscopic appearance
of the frozen heart preparation. The upper panel is of a
wafer of heart cut approximately midway between apex
and base. The left ventricle is open throughout, and
blood-free, allowing the ventricle to be frozen simultaneously from inside and out. Beginning at this level, the
right ventricle is partially closed. From this level to the
apex in this heart, the right ventricular wall abuts the
septal wall. Note the color differences in the arteries and
veins around the edges of the heart. The middle panel
demonstrates the striking color differences between artery
and vein in the frozen preparation. The lower panel is a
micrograph of a 30-/xm-thick frozen section which was
heat dried and then stained. In the center is an artery and
below this a vein. Even in these small vessels, approximately 28 (ira, arteries and veins are easily distinguished
by wall thickness.
A factorial analysis of variance was used to determine
whether differences in arterial and venous saturation
existed between and within ventricular walls. A value of
P <0.05 was accepted as significant, using the StudentNewman-Keuls procedure.15 In the studies of blood vessels traced through the left ventricular wall, a paired
Student f-test was used to compare initial and final subepicardial and subendocardial O2 saturation values. Regres-
MY0CARD1AL ARTERIOVENOUS O2 SATURATION/Wem and Sinha
Downloaded from http://circres.ahajournals.org/ by guest on June 14, 2017
'"ft"
FIGURE 2 The upper panel is a photograph of a frozen
section through the heart approximately midway between apex and base. The left ventricle is open and free
of blood while the right is partially collapsed in this
heart. Note the triads of arteries and veins around the
edge of the heart. The middle panel is a micrograph in
reflected light of an artery and vein. There is a striking
color difference as well as the arterial wall to aid in
distinguishing the vessels. The bar represents 250 tun
for the lower and middle panel. The lower panel is of a
30- pm-thick frozen section which was heat dried. The
preparation was stained with acid fuschin and methyl
blue, then photographed in transmitted light. A small
artery (upper) and vein in the center are shown.
121
CIRCULATION RESEARCH
122
VOL. 42, No. 1, JANUARY
1978
TABLE 1 Myocardial Arterial O2 Saturations (%)
Right
Septum
Left
Mean
90.4 ± 7.4
89.8 ± 8.6
90.2 ± 7.7
Apex
Base
91.0 ± 9.1
89.7 ± 5.4
90.9 ± 8.7
88.8 ±8.6
90.6 ± 7.5
89.8 ± 8.0
EPI
Middle
ENDO
91.2 ± 5.7
89.5 ± 8.9
Right
Middle
Left
90.6 ± 7.2
89.8 ± 10.2
89.1 ± 8.6
EPI
Middle
ENDO
91.8 ± 7.5
88.9 ± 7.6
89.7 ± 8.0
Results are expressed as mean ± SD. EPI = subepicardial; ENDO = subendocardial.
sion lines were constructed by least square analyses for
comparisons between blood vessel size and O2 saturation.
Results
Downloaded from http://circres.ahajournals.org/ by guest on June 14, 2017
Under our experimental conditions, arterial blood pressure was 134 ± 21/104 ± 16 mm Hg (mean ± SD) and
heart rate averaged 139.8 ± 25.0 beats/min. The arterial
Po2, was 82.4 ± 18.4 mm Hg; Pco2, 34.2 ± 6.5 mm Hg;
and pH 7.415 ± 0.057. The O2 saturation of the arterial
blood was 88.6 ± 13.3%. The hemoglobin concentration
averaged 13.5 ± 2.2 g/100 ml and the hematocrit was
43.2 ± 7.6%. These values are similar to those reported
by others with this type of preparation.1'*• "• 16 ' 17 No
changes in measured parameters were observed until the
hearts were fibrillated.
Myocardial Arterial O2 Saturation
The mean arterial saturation of 621 arteries measured
in all the hearts, regardless of size or position, was 90.1
± 7.9%. Comparisons were made between the right
ventricular, septal, and left ventricular walls (Table 1),
and no significant differences in arterial O2 saturation
were found. No difference in arterial O2 saturation was
observed regionally within any ventricular wall in comparisons between blood vessels found in the base half compared to the apical region (Table 1). No significant
differences in arterial saturations were found between
superficial and deep regions of the right and left ventricular free walls or between the right and left side of the
septal wall. However, the EPI regions had a slightly
higher saturation than the deeper, ENDO regions. Further studies were performed to determine whether a small
difference did, in fact, exist.
Arteries (n = 12) were followed through serial sections
of the left ventricular free wall for up to 7.5 mm to
determine whether arterial O2 saturation decreased with
depth. The arterial O2 saturation at the starting depth,
which ranged from 0.9 to 4.65 mm from the subepicardial
surface, was 96.9 ± 3.2%. At the greatest depth, each
vessel was followed (range 2.88-8.7 mm), the arterial O2
saturation averaged 94.5 ± 4.0%. These arterial O2
saturations were not different (paired Mest). The average
distance that vessels were followed was 4.2 mm.
Arterial O2 saturation was plotted against vessel size.
The percent difference from the average regional arterial
O2 saturation was compared to vessel diameter by a linear
regression plot. In all, 372 vessels were studied. No
correlation was found between inner vessel diameter and
arterial O2 saturation. In the studies of traced vessels,
initial diameter averaged 203 /xm (range, 77-912 /j.m)
and final diameter averaged 132 /xm (range, 43-298
/j.m). There was no significant decrease in saturation with
size. Further, when vessels of large and small initial size
were separated, no relationship between size and arterial
saturation existed. This is not to say that no arteries were
found with low O2 saturations. A few vessels in every
heart studied had arterial saturations in the range of 6070%. These vessels were of differing sizes and were
found throughout the septal, right ventricular, and left
ventricular walls.
Myocardial Venous O2 Saturation
The mean venous saturation of 612 veins, regardless of
size, measured in all hearts was 24.3 ± 10.1%. In three
hearts, coronary sinus O2 saturations were 35.8%, 23.1%,
and 40.0%, and the average left ventricular venous saturations were 31.4%, 29.6%, and 36.5%, respectively.
No significant differences were found between mean saturations of the right and left ventricular free and septal
walls (Table 2). Further, no differences were observed
TABLE 2 Myocardial Venous O2 Saturations (%)
Right
Septum
Left
Mean
22.6 ± 10.6
26.9 ± 9.8
23.1 ± 10.0
Apex
Base
25.2 ± 11.7
20.1 ± 9.1
25.3 + 9.8
28.5 ±9.8
23.4 + 10.1
22.8 ± 10.8
EPI
Middle
ENDO
25.6 ± 12.0
19.7 ± 8.4
Right
Middle
Left
27.9 ± 11.6
27.0 ± 9.4
25.7 ± 8.6
EPI
Middle
ENDO
Results are expressed as mean ± SD. EPI = subepicardial; ENDO = subendocardial.
* P 0.05 (Student-Newman-Keuls procedure).
29.8 ± 10.0*
23.1 ± 8.4*
16.4 ± 6.7*
MYOCARDIAL ARTER1OVENOUS O2 SATURATION/We«5 and Sinha
Downloaded from http://circres.ahajournals.org/ by guest on June 14, 2017
regionally between the apical and basal halves of any
ventricular wall. No differences were found in the septal
or right ventricular wall with depth, although the Endo
region of the right ventricle appeared to have a slightly
but insignificantly lower O2 saturation than the EPI.
In the left ventricular free wall, regional differences
with depth were observed. EPI venous O2 saturation was
significantly higher than that of the middle region and the
ENDO region by the Student-Newman-Keuls test. The
middle region was lower than the EPI and higher than
the ENDO region by the same test. The EPI venous O2
saturation averaged higher than the ENDO region in
every plug in all 12 hearts examined. Figure 3 shows
three histograms of the distribution of venous O2 saturations found in 234 blood vessles in six dogs in the EPI,
middle, and ENDO regions of the left ventricular free
wall. The histograms illustrate the shift to lower venous
O2 saturations observed with depth in the left ventricle.
Veins (n = 10) were followed in three hearts in serial
sections in the left ventricular free wall. Observation
began 0.9-4.65 mm in from the epicardial surface. The
greatest depth each vessel was followed ranged from 3.4
to 8.4 mm. There was a significant difference between
the superficial and deep saturation measurements, 45.7
± 13.5% vs. 34.5 ± 17.2%. This average difference is
slightly less than the average difference for all EPI vs.
ENDO venous O2 saturation differences.
Venous O2 saturation was plotted against vessel size in
the EPI and ENDO regions of the left ventricular free
wall. The percent difference from the average regional
venous saturation in a dog was compared to the vessel
diameter. No relation between vessel size and venous O2
saturation was found in the ENDO region. In the EPI, a
total of 107 veins were examined. There was a tendency
for larger veins to have a lower saturation, but this was
20H
123
110~
90-
is
•
—
S " 70-
S
—
1 50-
1—1
—i
a:
°
30-
q
1050
100
)0
Diameter of Veins
200+
(fi)
FIGURE 4 The standard deviation of the percent difference from
regional means of veins found in the left ventricle as compared to
their diameters. Variability of vessels below 75 \un appears greater
than in larger vessels.
not significant (r = 0.179). In the vessels traced through
serial sections, there was a decrease in diameter with
depth: initial average, 155 /j,m (range, 72-240 /nm) —
final average, 80 /urn (range, 48-110 /xm)-as there was
a decrease in venous O2 saturation with depth.
The relationship between the degree of variation in
venous O2 saturation and vessel diameter was studied.
The percent difference from the average regional venous
saturation in a heart was compared to vessel diameter in
212 veins. The results are shown in Figure 4. It can be
seen that the smaller vessels have a greater variability
than the larger vessels in terms of their venous O2 saturation. In vessels 75 fxm and greater, the standard deviation
in venous O2 saturations appears lower than that in
smaller vessels and is relatively constant.
Myocardial Arterial-Venous O2 Saturation Difference
ENDO
EPI
20
40
60
Venous 0 , Saturation (%{
FIGURE 3 Histograms of venous O 2 saturations found in small
veins in six hearts. These are of the superficial epicardial (EPI),
middle (MID), and deep endocardial (ENDO) region of the left
ventricular free wall. Note the shift in venous O2 saturations to
lower values with depth into the left ventricle.
The mean arterial-venous O2 saturation difference of a
ventricular wall and regional difference within a wall were
obtained from the average difference of all vessels in the
region or wall studied. No consideration was given to
vessel proximity. The average arterial-venous saturation
difference in all hearts examined was 66.0 ± 11.5%. No
differences existed in this parameter in comparisons between ventricular walls (Table 3). No significant variation
in arterial-venous O2 saturation differences was found in
regional comparisons between the base and apex in any
ventricular wall of the heart. No differences were found
between right ventricular EPI or ENDO regions or in the
septal wall between the right, middle, or left regions
(Table 3).
In the left ventricular free wall, the ENDO region had
a significantly higher arterial-venous O2 saturation difference (73.3%) than the middle (65.8%) or EPI (62.1%)
regions. This greater extraction of O2 in the deeper
ENDO region of the left ventricle was found in every
heart examined. Thus, differences in left ventricular O2
saturation with depth were also seen as greater O2 extractions.
CIRCULATION RESEARCH
124
VOL. 42, No. 1, JANUARY
1978
TABLE 3 Myocardial Arterial-Venous O2 Saturation Differences (%)
Right
Septum
Left
Mean
67.7 ± 10.2
62.9 ± 12.3
67.1 ± 1 1 . 2
Apex
Base
65.8 ± 11.4
69.6 ± 8.9
65.6 ± 12.6
60.3 ± 1 1 . 6
67.1 ± 11.3
67.0 ± 1 1 . 2
EPI
Middle
ENDO
65.6 ± 10.1
69.8 ± 10.3
Right
Middle
Left
62.7 ± 11.8
62.8 ± 14.2
63.4 ± 11.6
EPI
Middle
ENDO
62.1 ± 12.4
65.8 ± 9.9
73.3 ± 7.9*
Results are expressed as mean ± SD. EPI = subepicardial; ENDO = subendocardial.
• P 0.05 (Student-Newman-Keuls procedure).
Downloaded from http://circres.ahajournals.org/ by guest on June 14, 2017
In terms of vessel diameters, arteries that were significantly larger than veins were studied. The average diameter of arteries studied throughout the heart was 107 ± 61
/xm, whereas veins averaged 88 ± 43 /xm. In the left
ventricular free wall, significantly larger arteries and veins
in the EPI as compared to the ENDO region were
studied. EPI arteries averaged 130 ± 56 and ENDO
were 86 ± 28 /xm. Veins in the EPI averaged 113 ± 46
/urn in diameter and ENDO veins averaged 78 ± 30 /urn
in diameter.
Discussion
The method employed to determine the oxygenation of
hemoglobin in small arteries and veins in the canine
myocardium has been reported before.12'l3 The light
scattering in the frozen blood was corrected by reading
optical densities at three (560, 523, and 506 nm) different
wavelengths. Our method has an implied assumption that
only oxy- and deoxygenated hemoglobin species are present. We have shown that variations in the freezing rate
do not influence the determination of saturation. The
accuracy and repeatability of our measurements is better
than 3%. Our method is relatively independent of freezing
time, since no blood flow occurs during the freezing
process, and during freezing, large vessels do not undergo
a great deal of diffusive oxygen loss (Fig. 1). It is true
that there may be some loss of O2 at the capillaries due to
continuing, although steeply declining, metabolism. This
does not occur in larger vessels during freezing due to
greater wall thickness and diffusive barriers. Other methods have been employed to measure the O2 saturation of
frozen blood. Ours has the advantage of simplicity over
some18 and accuracy over others.7
No significant differences were found in either arterial
O2, venous O2, or arterial-venous O2 saturation between
the right, left, and septal walls of the heart. If differences
in O2 consumption exist between the ventricular walls,
they do not result in differences in ventricular wall O2
extraction. Such differences are likely, because the different walls perform at different work loads. A difference in
O2 consumption between the ventricular walls would have
to result, therefore, in a difference in blood flow between
the walls. Such differences in blood flow between the
right and left ventricles have been reported.1-17
The only significant regional difference in arterial or
venous saturation within any ventricular wall was that
venous O2 saturation decreased with depth into the left
ventricular free wall. This gradient in O2 extraction and
venous O2 saturation with depth in the left ventricular
free wall leads to the conclusion that the relationship
between O2 supply and demand is more precarious with
increasing depth. Tissue O2 tension measures, in part,
this relationship between O2 supply and demand. Gradients in tissue O2 tension have been reported in most2-:l
but not all19 polarographic studies such that EPI >
ENDO, and also in studies using mass spectroscopy.4 It
has been shown that the ratio of NAD+/NADH is smaller
in the ENDO region of the left ventricle.20 It also appears
in the ENDO that higher levels of anaerobic and lower
levels of aerobic enzyme activity exist.5-" Further, from
measurements of small-vessel blood content, it appears
that the ENDO region of the left ventricular free wall has
more open capillaries,21'22 also indicating a greater O2
need. The preponderance of evidence leads to the conclusion that under control conditions the EPI region has a
higher degree of relative oxygenation than the ENDO
region.
The difference in venous O2 saturation between EPI
and ENDO could be due to: (1) a diffusional loss of O2
from penetrating arteries, (2) a countercurrent arrangement of vessels which allows shunting O2 away from the
ENDO, (3) a lower ENDO blood flow with equal regional
O2 consumptions, and (4) a higher O2 consumption in the
ENDO.
No significant differences were found anywhere in the
heart with regard to arterial O2 saturation. Arterial O2
saturation was independent of wall, position, or depth,
although a few vessels were found with low O2 saturations.
This evidence appears to be quite different from that
reported in the hamster cheek pouch.8-9 In that preparation, arterial O2 saturation decreased significantly as vessel
size decreased. We have measured blood O2 saturation in
arterial vessels with internal diameters to just below 20
/Am and have found no significant decrease in O2 saturation. Duling and Pittman9 report arterial O2 saturations
of 58% and arterial PO2 values of 37 mm Hg in 19-/im
vessels whereas we find arterial O2 saturations of about
90% on similar size vessels. The difference between their
results and ours could be due to the major differences in
the preparations in which arterial O2 saturation was measured.
The gradient in venous O2 saturations in the left ven-
MYOCARDIAL ARTERIOVENOUS O2 SATURATION/lVeiss and SINHA
Downloaded from http://circres.ahajournals.org/ by guest on June 14, 2017
tricular free wall cannot be explained by a decrease in
arterial O2 saturation. The possible countercurrent arrangement of large vessels in the heart7 does not appear
to affect O2 delivery to the ENDO region. In the microvessels, the anatomy seems to preclude a great deal of
countercurrent flow.211
Regional blood flow to the ENDO region of the left
ventricular free wall has been reported to be lower than
that in the EPI, using various indicator washout techniques. On the other hand, measurements of the uptake
of various materials indicate a relative overperfusion of
the ENDO region. These studies have been reviewed.2'24
The distribution of radioactive microspheres in the heart
appears to be size-dependent. The smaller the microsphere diameter, the more nearly the EPI/ENDO blood
flow ratio approaches unity.25 Of late, the concensus
seems to be that blood flow gradients under control
conditions in the left ventricle are very small. It would
require an approximately 18% greater ENDO blood flow
to explain the difference in arterial-venous O2 extraction
without requiring a difference in regional O2 consumption.
Spotnitz et al.25 have demonstrated a greater subendocardial than subepicardial sarcomere length in diastole.
They also demonstrated a greater degree of subendocardial shortening in systole. This indicates a greater work
performed and hence higher O2 consumption in the
ENDO compared to the EPI region of the left ventricle.
It also gives credence to the determinations of a lower
relative aerobic level in the ENDO, even if regional
blood flow is uniform throughout the left ventricle. Furthermore, it has been shown by Krogh analysis that the
O2 consumption of the ENDO region appears to be
greater than that of the EPI region." O2 consumption
was calculated from measurements of regional blood flow,
relative tissue O2 tension, and small vessel blood content.
The data indicated that basal subendocardial metabolism
was 20-30% higher than subepicardial metabolic rate.
A recent report by Monroe et al.1" indicated no differences in average venous O2 saturation in the hearts of
anesthetized open-chest dogs between the EPI and
ENDO, contrary to that reported by the same group in
isolated hearts. This is, of course, also different from our
findings. There are several important differences between
the preparations and methods of measurements. The type
of anesthesia used was different. It has been shown that
high arterial O2 tension causes vasoconstriction in the
heart.27 Monroe et al. used a high O2 gas mixture, perhaps
causing greater vasoconstriction in the EPI region, thus
lowering these venous O2 saturation measurements. Further, our method of measurement is considerably more
accurate. Their results also were reported from measurements obtained in only four dogs, while we have used 12.
In our preparation, veins traced in serial sections inward
from the surface of the left ventricular free wall had
lower saturations the further in depth the vein was traced.
Venous diameter also decreased with depth. In a small
region such as the ENDO, however, no relation was
found between vessel diameter and venous O2 saturation.
In the EPI region this also was true, but there was some
tendency for larger veins to have a marginally lower O2
125
saturation. Larger veins in the EPI may be supplied in
part from deep within the heart where venous O2 saturations are lower. The possible statistical significance tendency is lost within the large variability of venous saturations observed, in the range of 0-65% (Fig. 3). This
great variability of venous O2 saturations within the heart
has also been reported by others.7-lfi'18
The most striking difference between small and large
veins is the degree of variability found in O2 saturations
(Fig. 4). Smaller veins are much more variable than large
veins. This great variability also has been shown in capillaries and small vessels in the rabbit myocardium by
Grunewald and Lubbers.18 The histogram of their data is
similar to ours. Our data suggest that large vessel venous
O2 saturations are averages of the very variable small
vessel O2 saturations. Such a degree of variability in
micro-areas of the heart is not surprising. Tissue O2
tension measurements within the same areas of the heart
show great variability even over small distances.2'3> l9
There also appears to be great variability in the number
of capillaries open in the heart under control conditions.28
Acknowledgments
The authors sincerely appreciate the excellent technical assistance
provided by Judith A. Neubauer.
References
1. Pitt A, Friesinger GC, Ross RS: Measurement of blood flow in
humans and dogs using 13;1Xenon technique. Cardiovasc Res 3: 100106,1969
2. Weiss HR: Control of myocardial oxygenation; effect of atrial pacing.
Microvasc Res 8: 362-376, 1974
3. Whalen, WJ, Nair P, Buerk D: Oxygen tension in the beating cat
heart in situ. In Oxygen Supply, edited by M Kessler et al. Baltimore,
University Park Press, 1973, pp 199-201
4. Loisance, DY, Owens G: A new device for recording pO2, pCO2,
and blood flow in focal areas of the myocardium. Am J Surg 12S:
496-500, 1973
5. Lundsgaard-Hansen P, Meyer C, Riedwyl H: Transmural gradient of
glycolytic enzyme activities in the left ventricular myocardium. I. The
normal state. Pfluegers Arch 297: 89-106, 1967.
6. Tota B: On the regional metabolism of beef heart ventricles. Acta
Physiol Scand 87: 289-295, 1973
7. Gamble WJ, LaFarge CG, Fyler DC, Weisul J, Monroe RG: Regional
coronary venous oxygen saturation and myocardial oxygen tension
following abrupt changes in ventricular pressure in the isolated dog
heart. Circ Res 34: 672-681, 1974
8. Duling BR, Berne RM: Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in
local regulation of blood flow. Circ Res 27: 669-678, 1970
9. Duling BR, Pittman RN: Oxygen tension; dependent or independent
variable in local control of blood flow? Fed Proc 34: 2012-2019,
1975
10. Grayson J, Davidson JW, Fitzgerald-Finch A, Scott C: The functional
morphology of the coronary microcirculation in the dog. Microcirculation in the dog. Microvasc Res 8: 20-43, 1974
11. Howe BB, Weiss HR, Wilkes SB, Winbury MM: Pentaerythritol
trinitrate and glyceryl trinitrate on intramyocardial oxygenation and
perfusion in the dog. Krogh analysis of transmural metabolism. Clin
Exp Pharmacol Physiol 2: 529-540, 1975
12. Sinha AK, Neubauer JA, Lipp JA, Weiss HR: Oxygen saturation
determination in frozen blood. Microvasc Res 10: 312-321, 1975
13. Sinha AK, Neubauer JA, Lipp JA, Weiss HR: Blood O2 saturation
determination in frozen tissue. Microvasc Res 14: 133-144, 1977
14. Van Slyke DD, Neill JM: The determination of gases in blood and
other solutions by vacuum extraction and manometric measurements
(1). J Biol Chem 61: 523-573, 1924
15. Zar JH: Biostatistical Analysis. Englewood Cliffs, N.J., PrenticeHall, 1974
16. Monroe RG, Gamble WJ, LaFarge CG, Benoualid H, Weisul J:
Transmural coronary venous O2 saturations in normal and isolated
hearts. Am J Physiol 228: 318-324,1975
17. Levy MN, Martins de Oliveira J: Regional distribution of myocardial
126
CIRCULATION RESEARCH
blood flow in the dog as determined by Rb™. Circ Res 9: 96-98, 1961
18. Grunewald WA, Lubbers DW: Die Bestimmung der intracapillaren
HbO2-Sattigung mit einer Kryo-mikrofotometrischen Methode angewandt am Myokard des Kaninchens. Pfluegers Arch 353: 255-273,
1975
19. Losse B, Schuchhardt S, Niederle N, Benzing H: The histogram of
local oxygen pressure (PO2) in the dog myocardium and the PO2
behavior during transitory change in oxygen administration. Adv Exp
Med Biol 37A: 535-540, 1973
20. Minamidate A, Takano S, Hashikawa I, Abiko Y: Transmural gradient of NAD+/NADH ratio in the canine left ventricular myocardium, and effects of coronary dilators on the transmural gradient. Jap
J Pharmacol 23: 126-128, 1975
21. Myers WW, Honig CR: Number and distribution of capillaries as
determinants of myocardial oxygen tension. Am J Physiol 207: 653660,1964
22. Weiss HR, Winbury MM: Nitroglycerin and chromonar on small
vessel blood content of the ventricular walls. Am J Physiol 226: 838843,1974
VOL. 42, No. 1, JANUARY
1978
23. Bassingthwaighte JB, Yipintsoi T, Harvey RB: Microvasculature of
the dog left ventricular myocardium. Microvasc Res 7: 229-249,
1974
24. Moir TW: Subendocardial distribution of coronary blood flow and
the effect of antianginal drugs. Circ Res 30: 621-627, 1972
25. Utley J, Carlson EL, Hoffman J1E, Martinez HM, Buckberg GD:
Total and regional myocardial blood flow measurements with 25 /*,
IS ft, 9 ft and filtered 1-10 ft diameter microspheres and antipyrine
in dogs and sheep. Circ Res 34: 391-405, 1974
26. Spotnitz HM, Sonnenblick EH, Spiro D: Relation of ultrastructure to
function in the intact heart: sarcomere structure relative to pressurevolume curves of intact left ventricles of dog and cat. Circ Res 18:
49-66, 1966
27. Ishikawa K, Lees T, Ganz W: Effect of oxygen on perfusion and
metabolism of the ischemic myocardium. J Appl Physiol 36: 56-59,
1974
28. Bourdeau-Martini J, Odoroff CL, Honig CR: Dual effect of oxygen
on magnitude and uniformity of coronary intercapillary distance. Am
J Physiol 226: 800-810, 1974
Downloaded from http://circres.ahajournals.org/ by guest on June 14, 2017
Pathophysiological Differences between Paired and
Communal Breeding of Male and Female
Sprague-Dawley Rats
BERNARD C. WEXLER AND BRUCE P. GREENBERG
SUMMARY Sexually mature, male and female Sprague-Dawley rats were housed in large communal breeding
cages or in smaller paired breeding cages. Virgin control rats of the same age were housed similarly but segregated
by sex. Breeders became obese, developed a fatty liver, and showed elevated levels of triglycerides, free fatty acids,
and cholesterol. Breeders had high blood pressure, enlarged hearts, hyperglycemia, and islet beta cell degranulation.
Serum enzymes, creatine phosphokinase, serum glutamic oxalo-pyruvic transaminase, serum glutamic pyruvic
transaminase, lactate dehydrogenase, and blood urea nitrogen levels were elevated in breeder rats. The adrenal
glands of male breeders appeared hyperactive; the adrenal glands of female breeders were Ihrombosed and
appeared to be hypoactive. Male breeder rats developed microscopic aortic lesions only; female breeders developed
advanced calcific aortic sclerosis. Male breeders kept in active stud service manifested the most abnormal metabolic
and pathophysiological changes. Female breeders developed similar pathophysiological changes after four pregnancies, irrespective of their paired or communal breeding environment. Virgin rats were normal regardless of housing
conditions. Our findings suggest that repeated breeding in male and female rats causes resetting of the hypothalamicpituitary-adrenal-gonadal axis. This may lead to disturbed hormonal and metabolic changes which culminate with
the development of accelerated cardiovascular degenerative changes.
MALE AND FEMALE rats of several strains spontaneously develop hyperglycemia, hyperlipidemia, hypertension, arteriosclerosis, and other degenerative changes
if they are bred actively and repeatedly.1"4 The severity of
these pathophysiological changes in repeatedly bred female rats appears to be related to the frequency and
number of pregnancies, as well as the number of young
suckled,5'6 and to the intensity of breeding activity in the
male.1"4 It is believed that repeated activation of the
From the May Institute for Medical Research of the Jewish Hospital
and Departments of Medicine and Pathology, University of Cincinnati
College of Medicine, Cincinnati, Ohio.
This work was supported in part by grants from the Southwestern Ohio
Heart Association and the National Institute of Aging (AG-585).
Address for reprints: Dr. Bernard C. Wexler, The May Institute for
Medical Research, 421 Ridgeway Avenue, Cincinnati, Ohio 45229.
Received January 24, 1977; accepted for publication August 15, 1977.
hypothalamic-pituitary-adrenal-gonadal axis associated
with the reproductive effort leads to resetting of hypothalamic-pituitary-interaction and disruption of normal hormonal processes that eventuates in a Cushingoid spectrum
of degenerative changes.7'8
We have found that longer periods of rest between
pregnancies or mating (in the male) will attenuate greatly
the usual incidence and severity of the Cushingoid degenerative changes which attend active and repeated breeding. Males placed in large breeder tanks, e.g., designed
to hold as many as 50 rats (40 females, 10 males) without
crowding, develop much more severe changes than those
placed in smaller laboratory cages for paired breeding. In
order to evaluate further these earlier findings, we compared the pathophysiological changes that occurred in
male and female breeder rats after four consecutive breedings during the time they were housed in a large, com-
Regional oxygen saturation of small arteries and veins in the canine myocardium.
H R Weiss and A K Sinha
Downloaded from http://circres.ahajournals.org/ by guest on June 14, 2017
Circ Res. 1978;42:119-126
doi: 10.1161/01.RES.42.1.119
Circulation Research is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231
Copyright © 1978 American Heart Association, Inc. All rights reserved.
Print ISSN: 0009-7330. Online ISSN: 1524-4571
The online version of this article, along with updated information and services, is located on the
World Wide Web at:
http://circres.ahajournals.org/content/42/1/119
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in
Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the
Editorial Office. Once the online version of the published article for which permission is being requested is
located, click Request Permissions in the middle column of the Web page under Services. Further information
about this process is available in the Permissions and Rights Question and Answer document.
Reprints: Information about reprints can be found online at:
http://www.lww.com/reprints
Subscriptions: Information about subscribing to Circulation Research is online at:
http://circres.ahajournals.org//subscriptions/