* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Homework Nov 28-Dec 2 - Avery County Schools
Southern Ocean wikipedia , lookup
Indian Ocean Research Group wikipedia , lookup
Abyssal plain wikipedia , lookup
Deep sea fish wikipedia , lookup
Indian Ocean wikipedia , lookup
Marine pollution wikipedia , lookup
Marine biology wikipedia , lookup
Arctic Ocean wikipedia , lookup
Ocean acidification wikipedia , lookup
Marine habitats wikipedia , lookup
Effects of global warming on oceans wikipedia , lookup
Physical oceanography wikipedia , lookup
Ecosystem of the North Pacific Subtropical Gyre wikipedia , lookup
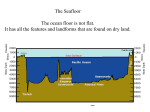
Homework Nov 28-Dec 2 H H O Monday : Water Properties Review When water molecules stick to each other________________ When water molecules stick to something else ______________ Property of water meaning it has opposite charges on each side of the molecule, allows water molecules to “stick” together________________ Skin-like layer on water’s surface that allows paper clips to float_____________ What is the chemical formula for WATER?____________ The Density of water is ________ Tuesday: Chemical reactions Word Bank: subscript, reactants, products, coefficient, law of conservation of matter, law of conservation of mass, atoms. The _______ of ___________ of ______________ states that Matter cannot be created or destroyed. This is supported by the number of ________ correctly balanced in a chemical equation. The ______________ appear on the left side of the equation. The ___________ appear on the right side of the equation. The small number, the ___________ in a molecular formula shows the number of atoms. The large number, ____________ shows the number of molecules. The _____________of ____________of _____________states that the mass of the ______________ must equal the mass of the _______________. Label the Reactants, Products, subscripts, and coefficients. 2H2 (g) + O2 (g) 2H20 (l) Look at the following reaction: 2Na (s) + Cl2 (g) 2NaCl (s) If there is 40 grams of Na and 20 grams of Cl . What is the mass of the products? Balance the following equation: HCl (l) + Mg (s) MgCl2 (s) + H2 (g) Wednesday Ocean life: 1). 2). 3). 4). 5). 6). 7). 8). 9). Term used to describe “free swimming” animals in the ocean _______________ Term used to describe “bottom dwelling” organisms ______________ Ocean current that brings cold deep water to the ocean surface, _____________ The place where ocean (salt) water meets fresh water _______________ Area of ocean between high and low tide, Examples of organisms are: crabs, starfish, anemones______________ Zone of ocean between low tide and edge of continental shelf, organisms are: lobster, seahorse____________ Zone of ocean goes from continental shelf to open ocean. Organisms are: jellyfish, tuna, dolphins.__________________ Zone of ocean goes from continental slope to 2000m depth organisms are: octopus, squid __________________ Deepest ocean zone. No sunlight. Organisms: devilfish, anglerfish _________________ Thursday : Periodic Table Use the background color on the periodic table to identify each element as a Metal (M), Nonmetal (NM), or Metalloid (ML). All of these elements are in the first 5 periods of the periodic table. 1. Magnesium _____ 6. Vanadium_____ 2. Carbon ____ 7. Boron_____ 3. Neon_____ 8. Aluminum_____ 4. Silicon_____ 9. Sulfur_____ 5. Lithium_____ 10. Zinc_____ QUIZ FRIDAY!!! STUDY, STUDY, STUDY THIS HOMEWORK SHEET!!!