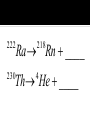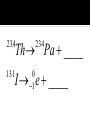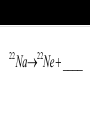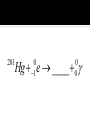* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download nuclear chemistry notes 1
Survey
Document related concepts
Nuclear fusion wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Valley of stability wikipedia , lookup
Transcript
Elements 84-110 are radioactive. Elements 93-110 are man-made radioactive elements. Since these man-made atoms are so big, they can be created by combining two smaller atoms together. The protons and neutrons combine into one large nucleus. When studying nuclear chemistry, we use the standard symbol notation. A X Z X is the symbol for the atom A is the mass number (protons + neutrons) Z is the atomic number (protons) Smaller atoms want to have close to the same number of protons and neutrons. This is when they are the most stable. Large atoms with around 83 protons or more are so large that they too are unstable. If atoms are too unstable, they will decompose, or radioactively decay, releasing different types of particles in order to become more stable. Common for heavy radioactive nuclei (above atomic number 83) Alpha particle is produced 4 2 He or 4 2 Not very penetrating; cannot pierce skin; shielded by paper or clothing 222 Ra Rn ____ 218 Th He ____ 230 4 Common for elements with high neutron to proton ratio Beta particle produced Beta particle is basically an electron (very little mass) 0 0 or e 1 1 More penetrating than alpha; can get through 1 cm of flesh; may pass through clothing & damaged skin Th Pa ____ 234 234 I e ____ 131 0 1 High energy LIGHT; NOT a particle Only change in nucleus is in amount of energy 0 0 Most penetrating of all; can pass through the body & cause damage shielded only by lead and/or concrete U He Th ____ 238 4 234 Common for lighter elements with low neutron to proton ratio Particle has same mass as electron but OPPOSITE CHARGE 0 1 e 22 Na Ne ____ 22 Common for larger elements with a low neutron to proton ratio A nucleus captures one of its OWN electrons Captured electron joins with a proton in the nucleus to form a neutron Gamma rays are always produced with electron caputre Hg e ____ 201 0 1 0 0