* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Activity 3
Metalloprotein wikipedia , lookup
Point mutation wikipedia , lookup
Targeted temperature management wikipedia , lookup
Biochemistry wikipedia , lookup
Optical tweezers wikipedia , lookup
Protein structure prediction wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Protein purification wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Western blot wikipedia , lookup
Human digestive system wikipedia , lookup
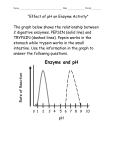
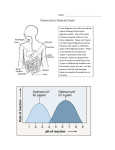
Group 1: Aguila, Alog, Alejandro, Almajar, Angeles, Araño, Balictar, Buemio, De los Arcos, Escobillo, Manuel, Peña, Rabanal, Rivera, Ronquillo, Siazon, Sutingco, Tagalog, Tamayo, C. Uy, Yeo, Yu Protein Digestion by Pepsin A protein-digesting enzyme • Produced by chief cells of the stomach glands Hydrolyzes proteins to small fragments • Amino acids and some polypeptides 2 types of glands in the stomach mucosa: • Oxyntic (acid-forming) glands aka gastric glands Inside surfaces of the body and fundus Secrete Hydrochloric acid, pepsinogen, intrinsic factor, mucus, gastrin Composed of 3 types of cells Mucous neck cells: secrete mucous Peptic chief cells: secrete pepsinogen Parietal (oxyntic) cells: secrete HCl and intrinsic factor • Pyloric glands Pepsinogen: inactive form of Pepsin • Becomes activated into pepsin when it comes into contact with Hydrochloric acid Pepsin • An active proteolytic enzyme in a highly acidic medium Optimum pH 1.8 – 3.5 Above pH of 5: almost no proteolytic activity and may become completely inactivated Requires stomach juices to be acidic for it to be active HCl secreted by parietal (oxyntic) cells in glands • When mixed with stomach contents, pH becomes 2.0-3.0 (highly favorable for pepsin activity) Pepsin can digest collagen • Important for digestion of meat and other meat proteins Only initiates protein digestion • 20% of total protein digestion • Converts protein to: Proteases Peptones Few polypeptides • Breaks down protein by hydrolysis at peptide linkages between amino acids Protein Digestion by Pepsin BAPNA: synthetic protein • Transparent and colorless in solution • Will turn yellow if active pepsin (or any protein digesting enzyme) is present Pepsin will digest BAPNA Pepsin Incubation • Boil Test tube 1 Pepsin Assay • Spectrophotometer: to measure optical density Incubation Temperature: 37°C Incubation Time: 60 minutes Incubation Temperature: 37°C Incubation Time: 30 minutes Incubation Temperature: 10°C Incubation Time: 60 minutes 1. Which pH provided the highest pepsin activity? How does this correlate to the location of pepsin in the body? pH 2.0 Pepsin obtains optimal activity in a highly acidic medium (pH 1.8-3.5). When pH exceeds 5.0, very little or no proteolytic activity will take place. This pH correlates with the pH in the stomach which has an acidic pH of 2.0-3.0 when HCl mixes with stomach contents 2. Would pepsin be active in the mouth? Explain. No. The normal pH of the mouth stays close to neutral (7.0). This pH however, can change temporarily when certain foods are digested. The pH of the mouth does not reach the ideal acidity in order for pepsin activity to take place (pH 1.8 – 3.5). 3. How did the results of tube 1 compare with those of tube 2? Test tube 1 displayed an optical density of 0.00 while test tube 2 displayed an optical density of 0.40. The solution in test tube 1 remained clear whereas the solution in test tube 2 turned yellow, evidence that BAPNA in test tube 2 was digested. 4. Tubes 1 and 2 contained the same substances. Explain why their optical density measurements were different. The boiling of the solution in test tube 1 gave rise to the difference in optical density measurements between tubes 1 and 2. The boiling of the solution in test tube 1 caused the denaturing of pepsin, rendering this inactive and unable to digest BAPNA. 5. Did the pepsin or deionized water contain any contaminating digested BAPNA? Which tubes confirm this? • No. The pepsin or deionized water did not contain contaminating digested BAPNA. Test tubes 3 and 4 confirm this with 0.00 optical density. 6. What do you think would happen if you reduced the incubation time to 30 minutes? How did this affect optical density results? • Reducing the incubation time to 30 minutes reduced the optical density due to the reduced amount of time for digestion of BAPNA. 7. What do you think would happen if you decreased the temperature to 10°C? What effect would this have on pepsin activity? Why? Decreasing the temperature to 10°C would decrease pepsin activity and would decrease the amount of digested BAPNA. Decreasing the temperature would, in effect, decrease optical density. Pepsin works best at body temperature (~37°C) What effect did boiling have on pepsin? Boiling led to the denaturation of pepsin rendering this inactive. What is the substrate in the experiment? • The substrate used in the experiment was BAPNA BAPNA: Nα-benzoyl-DL-arginine-p-nitroaniline What was the significance of using 37°C for incubation? • 37°C was used for incubation because the experiment tried to simulate the activity of pepsin in the body. 37°C is representative of normal body temperature. Guyton, Arthur, and John Hall. Textbook of Medical Physiology. 11th ed. Philadelphia, Pennsylvania: Elsevier Saunders, 2006. Print. "Pepsin." Enzyme Explorer. 2009. Sigma-Aldrich Co., Web. 25 Nov 2009. <http://www.sigmaaldrich.com/lifescience/metabolomics/enzymeexplorer/analytical-enzymes/pepsin.html>.