* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Alkanes - Warren County Schools
Survey
Document related concepts
Transcript
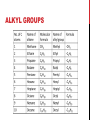
ALKANES HYDROCARBONS HISTORY OF ORGANIC Early 19th century chemists were only familiar with inorganic, ionic compounds. They were baffled by the fact that several compounds that were found in fruits and vegetables were composed of many carbon, hydrogen, nitrogen and oxygen atoms in different combinations were stable. The “vital force theory” grew out of the idea that these molecules could only come from living organisms. In 1828, Wohler did an experiment that destroyed this idea…he made urea from ammonia and cyanic acid. Now, we know that there are virtually a limitless number of organic compounds that can be synthesized. ORGANIC COMPOUNDS Properties of organic substances are related to their structure. Most compounds contain carbon, hydrogen and any of the other following elements: oxygen, nitrogen, phosphorus, sulfur and the halogens. Many of these atoms follow typical bonding patterns: C has 4 bonds and no lone pairs. N has 3 bonds and 1 lone pair. O and S have 2 bonds and 2 lone pairs. H has 1 bond and no lone pairs Halogens (F, Cl, Br, I) have 1 bond and 3 lone pairs. HYDROCARBONS 2 categories: 1) Aromatic- compounds with carbon and hydrogen that contain benzene rings 2) Aliphatic- compounds with carbon and hydrogen that are not aromatic (alkanes, alkenes, alkynes, and cycloalkanes) Benzene ALKANES Alkane is the term used for saturated hydrocarbons, meaning that no multiple bonds exist between carbons. These molecules are nonpolar, due to electronegativity differences. Weak intermolecular forces exist, and so these molecules have relatively low boiling points. Typical alkanes follow this pattern for formulas: CnH2n+2 ISOMERS In some cases, it is possible to draw molecules with 2 different structures that have the same chemical formula. That is an isomer, by definition. Isomers are common among organic compounds. Examples: n-butane and isobutane IUPAC NAMING IUPAC stands for International Union of Pure and Applied Chemistry. This group of professional chemists set guidelines for naming chemical compounds. Different naming procedures are used for organic molecules than what are used for inorganic compounds. In order to name compounds, it is important to recognize many common alkyl groups. Alkyl refers to a group with one less hydrogen than an alkane (CnH2n+1). IUPAC NAMING Three prefixes used commonly indicate structural info: Iso- has this structure at one end of a carbon chain Sec- (for secondary) Tert- or t- (for tertiary) PRIMARY, SECONDARY AND TERTIARY Primary (1°) refers to a carbon that is bonded to 1 other carbon. Secondary (2°) refers to a carbon that is bonded to 2 other carbons. Tertiary (3°) refers to a carbon that is bonded to 3 other carbons. ALKYL GROUPS RULES FOR NAMING ALKANES 1) Select the longest continuous chain of carbon atoms as the parent compound, and consider all alkyl groups attached to it as branch chains that have replaced hydrogen atoms of the parent hydrocarbon. (The name of the alkane consists of the name of the parent compound prefixed by the alkyl groups attached to it.) 2) Number the carbon atoms in the parent carbon chain from one end to the other starting with the end closest to the first carbon atom that has a branch chain. 3) Name each branch-chain alkyl group and designate its position on the parent carbon chain by a number. 4) When the same branch alkyl group occurs more than once, indicate this by a prefix (di, tri, tetra, etc) written in front of the alkyl group name. 5) The numbers indicating the carbons that the groups are on are separated by commas, then a hyphen in front of the group name. SUMMARY 1) Find the longest chain! Be sure to check groups attached…they may be part of the main chain! 2) Number the carbons from the end where an attached group is nearest. 3) Use numbers to tell which carbon the group is attached to. 4) Use prefixes if there are more than one of the same group. 5) Always list branches in alphabetical order. LINE STRUCTURE In organic, we often write structure with lines to look less confusing than structural formulas. Remember.. Each point, or end of a line segment represents a carbon atom. It is assumed that as many hydrogens are attached as possible to give the carbon an octet. EXAMPLES Write the structural formula for the line structures shown. 1) 2) PRACTICE Name the following: Draw the following: 1) 3) 2-methylhexane 4) 3,4-dibromoheptane 2) 5) 2-chloro-3-ethylpentane REACTIONS OF CARBON 1) Oxidation-reduction reactionWhen carbon atoms are oxidized (lose electrons), they often form additional bonds to oxygen. When carbon atoms are reduced, they often form additional bonds to hydrogen. 2) Substitution reactionMany involve halogens… Methane gas plus bromine liquid REACTIONS OF CARBON 3) Elimination reactions (often called dehydrogenation)Single reactant is split into two products, and one is eliminated. These reactions form multiple bonds. 4) Addition reactions- reverse of an elimination reaction…two reactants come together to form one product. Ethene + HBr SOURCES OF ALKANES Natural gas and petroleum are the two main sources of alkanes. Main component of natural gas is methane (8095%). Petroleum is refined into useful products like gasoline, kerosene, jet fuel, diesel fuel, paraffin wax, and petroleum jelly. Alternative fuel sources must be developed because these sources will run out and are already in shorter supply. CYCLOALKANES General formula- CnH2n Named by adding cyclo to the main chain name… For example, a six carbon cyclic alkane is called cyclohexane. Can you draw it? Cyclopropane and cyclobutane are more reactive than their main chain constituents, but the longer chains are similarly reactive as their cyclic cousins. CYCLOPROPANE AND CYCLOBUTANE Normally carbon molecules have a tetrahedral geometry with a 109.5 degree bond angle. However in cyclopropane, the angles are strained and forced to be 60 degrees! Cyclobutane forces carbon to have bond angles of 90 degrees, thus it is also strained. The strain causes it to be really reactive because the bonds are weakened, so they are easily broken as a result. OTHER THINGS ABOUT RINGS The conformation, or shape in 3-D, of rings will assume the most stable arrangement possible. This depends on the distance between hydrogens. Equatorial –(blue) the hydrogens in the same plane as carbon Axial- (red)the hydrogens are at right angles to the carbon CONFORMATIONS OF CYCLOHEXANE There are 2 conformations of cyclohexane. Chair and Boatchair is most stable NAMING CYCLOALKANES The base of the name of a cycloalkane is … Cyclo- plus the root that describes the number of carbons present in the ring. If they are substituted, meaning they have a group branched off of the ring, then the carbons are numbered either clockwise or counterclockwise so that the branches are off of the lowest numbered carbons possible.