* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Posterior Pericardial Ascending-to-Descending Aortic
History of invasive and interventional cardiology wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Coronary artery disease wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Marfan syndrome wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Turner syndrome wikipedia , lookup
Aortic stenosis wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
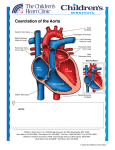
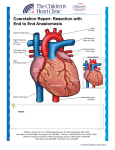
Posterior Pericardial Ascending-to-Descending Aortic Bypass An Alternative Surgical Approach for Complex Coarctation of the Aorta Heidi M. Connolly, MD; Hartzell V. Schaff, MD; Uzi Izhar, MD; Joseph A. Dearani, MD; Carole A. Warnes, MD; Thomas A. Orszulak, MD Downloaded from http://circ.ahajournals.org/ by guest on June 12, 2017 Background—Coarctation of the aorta is commonly associated with recoarctation or additional cardiovascular disorders that require intervention. The best surgical approach in such patients is uncertain. Ascending-to-descending aortic bypass graft via the posterior pericardium (CoA bypass) allows simultaneous intracardiac repair or an alternative approach for the patient with complex coarctation. Methods and Results—Between 1985 and 2000, 18 patients (13 males and 5 females, mean age 43⫾13 years) with coarctation of the aorta underwent CoA bypass through median sternotomy. Before operation, average New York Heart Association class was II (range I to IV), and 15 patients (83%) had systemic hypertension. One or more previous cardiovascular operations had been performed in 12 patients (67%); 10 patients had ⱖ1 prior coarctation repair. Two patients had prior noncoarctation cardiovascular surgery. Concomitant procedures performed in 14 patients (78%) included the following: aortic valve replacement in 9; coronary artery bypass surgery in 3; mitral valve repair in 2; and septal myectomy, mitral valve replacement, aortoplasty, subaortic stenosis resection, ventricular septal defect closure, and ascending aorta replacement in 1 patient each. All patients survived the operation and were alive with patent CoA bypass at a mean follow-up of 45 months. No graft-related complications occurred, and there were no instances of stroke or paraplegia. Systolic blood pressure fell from 159 mm Hg before surgery to 125 mm Hg after surgery. Conclusions—CoA bypass via median sternotomy can be performed with low morbidity and mortality. Although management must be individualized, extra-anatomic CoA bypass via the posterior pericardium is an excellent single-stage approach for patients with complex coarctation or recoarctation and concomitant cardiovascular disorders. (Circulation. 2001;104[suppl 1]:I-133-I-137.) Key Words: heart defects, congenital 䡲 coarctation 䡲 bypass 䡲 aorta 䡲 surgery C reviews indications, techniques, and results of the posterior pericardial approach to the descending aorta in 18 consecutive patients with coarctation of the aorta. oarctation of the aorta is commonly associated with congenital and acquired cardiac pathology that may require surgical intervention.1,2 In addition, 5% to 30% of patients with previous coarctation repair have recoarctation and require reintervention.3–5 Adult patients with native or recurrent coarctation with or without associated intracardiac disease pose a surgical challenge. There is no consensus on the optimal approach for these patients. Several extraanatomic bypass grafting techniques have been described, including methods in which the distal anastomosis is performed on the descending thoracic aorta, supraceliac abdominal aorta, or the infrarenal abdominal aorta.3,4,6 –9 In 1980, Vijayanagar et al10 described exposure of the descending thoracic aorta through a median sternotomy and posterior pericardium. This procedure allowed bypass of coarctation with concomitant aortic valve replacement. Additional reports detail modifications of this procedure for complex forms of aortic coarctation.4,11,12 The present study Methods We reviewed the clinical, surgical, and follow-up records of 18 patients with coarctation of the aorta who underwent extra-anatomic ascending-to-descending aortic bypass grafting through a median sternotomy between December 1985 and December 2000. Clinical characteristics are outlined in Table 1. The ages of the 13 males and 5 females ranged from 15 to 66 (mean 43⫾13) years. All patients underwent preoperative transthoracic echocardiography. Additional imaging studies included aortography in 11 patients, coronary angiography in 10 patients, and MRI of the descending thoracic aorta in 6 patients. Twelve of the 18 patients (67%) had previously undergone cardiac or thoracic aortic operations (Table 2). Concomitant cardiovascular procedures were performed in 14 patients (78%) (Table 3). Six patients had no prior cardiovascular surgery. From the Division of Cardiovascular Diseases (H.M.C., C.A.W.) and the Division of Cardiovascular Surgery (H.V.S., U.I., J.A.D., T.A.O.), Mayo Clinic, Rochester, Minn. Dr Izhar is now at Hebrew University Hadassah Medical Center, Jerusalem, Israel. Reprint requests to Dr Heidi M. Connolly, Division of Cardiovascular Diseases, Mayo Clinic, 200 First St SW, Rochester, MN 55905. E-mail [email protected] © 2001 American Heart Association, Inc. Circulation is available at http://www.circulationaha.org I-133 I-134 Circulation September 18, 2001 TABLE 1. Demographic Characteristics of the Study Group Characteristic No. of Patients or Value Mean age (range), y TABLE 2. % Operation 43 (15–66) Female Previous operation 10 Aortic valvotomy 1* 13 72 AVR 1* 5 28 CABG 1 12 67 SubAS resection 1† PDA ligation 1† Cardiac 3* Thoracic 10* Hypertension 15 83 3 17 I 6 33 II 8 44 III 3 17 IV 1 6 CAD No. of Patients Repair of coarctation Sex Male Previous Cardiac or Thoracic Operations AVR indicates aortic valve replacement; SubAS, subaortic stenosis; and PDA, patent ductus arteriosus. *Same patient. †Same patient. Preoperative NYHA class Downloaded from http://circ.ahajournals.org/ by guest on June 12, 2017 CAD indicates coronary artery disease; NYHA, New York Heart Association. *Two patients had ⬎1 operation. Indications for Extra-Anatomic Aortic Bypass Grafting Extra-anatomic aortic bypass grafting was performed for 2 indications in this series. The first indication was coarctation or recoarctation and associated cardiac problems that required repair through median sternotomy (group 1, patients 1 to 4, 6, 8, 10 to 12, 14 to 18; Table 3). The second indication was complex coarctation or recoarctation, for which extra-anatomic bypass grafting was chosen because of the anticipated difficulties with direct anatomic repair (group 2, patients 5, 7, 9, and 13; Table 3). Complex coarctation was defined as a long coarctation or recoarctation segment, a pseudoaneurysm at a previous aortic isthmus suture line, or concomitant hypoplasia of the aortic arch. TABLE 3. Patient The preoperative diagnoses among patients in group 1 included recurrent coarctation of the aorta (n⫽7) and coarctation of the aorta (n⫽6). Associated cardiovascular problems included aortic valve stenosis (n⫽5), aortic valve regurgitation (n⫽4), coronary artery disease (n⫽3), mitral valve regurgitation (n⫽3), ascending aortic dilation (n⫽2), subaortic stenosis (n⫽2), ventricular septal defect (n⫽1), and hypertrophic obstructive cardiomyopathy (n⫽1). The preoperative diagnoses among patients in group 2 included recoarctation of the aorta (n⫽2), hypoplastic transverse aorta associated with coarctation (n⫽1), and pseudoaneurysm formation after 2 previous coarctation repairs (n⫽1). Operative Technique The surgical approach used in all patients was the median sternotomy. After aortic and right atrial or bicaval cannulation, hypothermic (16°C to 34°C) cardiopulmonary bypass (mean time 118⫾51 minutes, range 18 to 217 minutes) was established. Arterial cannulation was accomplished by using the ascending aorta in all but 1 patient, in whom femoral artery cannulation was used. Cephalad retraction of the heart and longitudinal pericardial incision directly over the descending thoracic aorta allowed exposure of the aorta through the posterior pericardium in all patients. The aorta was dissected to allow Preoperative Diagnosis and Concomitant Procedures Age, y Diagnosis Additional Procedure AVR 1 47 Recoarctation, aortic stenosis 2 39 Recoarctation, aortic regurgitation AVR 3 15 Recoarctation, mitral regurgitation MVRe 4 59 Recoarctation, CAD CABG 5 50 Recoarctation 6 66 Recoarctation, aortic stenosis, mitral regurgitation, HCM AVR, MVR, septal myectomy 7 46 False aneurysm, status postrepair coarctation ⫻2 LCCA-LSCA bypass graft 8 41 Recoarctation, aortic regurgitation AVR 9 31 Recoarctation 10 49 Recoarctation, aortic stenosis, CAD AVR, CABG 11 48 Coarctation, VSD VSD closure 12 28 Coarctation, subaortic stenosis, aortic regurgitation Subaortic stenosis resection, AVR 13 44 Coarctation, hypoplasia of transverse arch 14 55 Interrupted arch, ascending aneurysm, aortic regurgitation Aortic root replacement, AVR 15 40 Coarctation, subaortic stenosis Subaortic stenosis resection 16 44 Coarctation, mitral regurgitation MVR 17 25 Coarctation, prosthetic aortic stenosis AVR 18 55 Coarctation, CAD CABG HCM indicates hypertrophic cardiomyopathy; VSD, ventricular septal defect; MVRe, mitral valve replacement; MVR, mitral valve repair; LCCA, left common carotid artery; and LSCA, left subclavian artery. Connolly et al Coarctation of the Aorta I-135 Figure 2. Postoperative MRI of patent ascending-to-descending aortic bypass graft (patient 7, Table 3). S indicates superior; I, inferior; R, right; and L, left. Downloaded from http://circ.ahajournals.org/ by guest on June 12, 2017 (patient 7, Table 3). The proximal anastomosis was completed after the cardiac operation. Results Figure 1. Drawing of ascending (ASC)-to-descending (DESC) aortic bypass via posterior pericardium (patient 10, Table 3). Simultaneous aortic valve replacement and CABG was performed. RA indicates right atrium; IVC, inferior vena cava; L. IMA, left internal mammary artery; LAD, left anterior descending coronary artery; Coarct, coarctation; SVG, saphenons vein graft; and DIAG, diagonal coronary artery. placement of a partially occluding vascular clamp. This was used to control the descending aorta, and the end-to-side Dacron graft-toaorta anastomosis was made with continuous 4-0 polypropylene suture. Unnecessary dissection in the region of the esophagus was avoided. The graft was directed anterior to the esophagus and routed posterior to the inferior vena cava but anterior to the right inferior pulmonary vein (Figure 1). The graft was led around the right atrium and anastomosed to the right lateral aspect of the ascending aorta by using a side-biting clamp. Bypass graft sizes were 20, 22, or 24 mm in 16 patients; 2 patients (patients 3 and 13, Table 3) with small descending aortas received a 16- and 18-mm graft, respectively. Cardiopulmonary bypass was used in all patients in this series. Concomitant intracardiac procedures or CABG were accomplished after performing the distal anastomosis of the graft to the descending aorta and after introduction of cardiac arrest with antegrade coldblood cardioplegia and aortic cross clamping. The mean cross-clamp time was 52⫾29 (range 23 to 128) minutes. Deep hypothermia and total circulatory arrest (mean 20⫾9 minutes, range 14 to 34 minutes; temperature 18°C to 20°C) was used in 4 patients. This was required to facilitate exposure and construction of the distal anastomosis to the descending aorta in 3 patients (patients 1, 3, and 13; Table 3) and to exclude a false aneurysm of the aortic isthmus in 1 patient (patient 7, Table 3). The diseased segment of the descending thoracic aorta was excluded from circulation in the patient with a false aneurysm No early or late deaths occurred. Early postoperative morbidity included reexploration for control of bleeding from an intercostal artery in 1 patient, transient atrial fibrillation in 2 patients, and permanent pacemaker implantation in the patient who underwent ventricular septal defect closure. There were no instances of postoperative paraplegia, and there were no permanent abnormalities in the neurological examination. The mean hospital stay was 8⫾3 (range 5 to 17) days. Follow-up was 100% complete and extended to a maximum of 13 years (mean 45⫾48 months). Reoperation was required in 1 patient for a perivalvular leak 8 months after mitral valve replacement and coarctation bypass (patient 3, Table 3). No late graft-related complications or reoperations occurred. At follow-up, echocardiography demonstrated patency of all grafts. Additional postoperative imaging studies demonstrated graft patency in 4 patients, magnetic resonance angiography in 1 (patient 7, Table 3, Figure 2), and computerized tomography in 3 (patients 8, 10, and 14; Table 3). Systolic blood pressure decreased after surgery (158 mm Hg for average preoperative blood pressure versus 125 mm Hg for average postoperative blood pressure). Overall, left ventricular ejection fraction did not change after surgery but did improve substantially in 2 patients (patients 4 and 10, Table 3) who had severe left ventricular dysfunction before surgery (from 21% to 58% and from 35% to 59%, respectively). Discussion Complex forms of coarctation have been surgically approached by using anatomic repair and extra-anatomic bypass grafting.3,4,6 –10,13 Anatomic repair may be complicated by the need for extensive mobilization of the aorta, control of collateral blood vessels, the possibility of parenchymal lung injury, damage to the recurrent laryngeal or phrenic nerves, chylothorax, and spinal cord ischemia.14 The most feared complication of aortic surgery is paraplegia and risk of spinal I-136 Circulation September 18, 2001 Downloaded from http://circ.ahajournals.org/ by guest on June 12, 2017 cord injury. The risk of these complications increase with prolonged aortic cross-clamp time and older age.14 Nonsurgical interventional procedures are possible for native or recurrent coarctation; however, these procedures are not feasible or desirable in all patients,15 and long-term outcome data are not available. The best management for patients with coarctation and recoarctation remains uncertain. Several reports6,16 describe the outcome of ascending aorta–to– descending aorta bypass grafting through combined left thoracotomy and median sternotomy. Sweeney et al4 described 16 patients who had a bypass graft placed for recoarctation; in 4, the graft from the ascending to the descending aorta was placed through a median sternotomy. The 2 surgical approaches for patients with coarctation of the aorta and associated cardiac defects that require repair include a 1-stage simultaneous correction of both lesions through a median sternotomy4,9,10,14,17–19 and a 2-stage repair through median sternotomy and lateral thoracotomy.20,21 In 1980, Vijayanagar et al10 described an adult patient with coarctation of the aorta and severe aortic valve regurgitation. Ascending aorta–to– descending aorta bypass was performed by exposing the descending thoracic aorta through the posterior pericardium and placing the graft around the left margin of the heart. The proximal anastomosis was made to the left lateral aspect of the ascending aorta. Powell et al22 described a modification of this technique, which routed the graft around the right margin of the heart. In this modification, the proximal anastomosis was made to the right lateral aspect of the ascending aorta. Since these initial descriptions, additional investigators have used the posterior pericardial approach to the descending aorta.4,9 –12,14,17–19 A recent report by Izhar et al23 from our institution included 10 of the coarctation patients reported in the present series but also included 7 patients with aortic disorders unrelated to coarctation. In the present series, the posterior pericardial approach was used in 18 consecutive adolescent and adult patients presenting with coarctation or recoarctation with or without associated cardiac disorders. The incidence of recurrent coarctation of the aorta is between 5% and 30%,3–5 and no single surgical method is applicable to all patients.3,4,8,13 When coarctation or recoarctation is associated with cardiac defects that require repair, a 1-stage approach using cardiopulmonary bypass and coarctation bypass grafting through the posterior pericardium is a safe surgical alternative. This combined approach avoids the potential complications of anatomic repair and reoperation and allows a concomitant procedure through the same incision. The graft is placed between the inferior vena cava and the right inferior pulmonary vein, a route that keeps the graft in a posterior location avoiding compression of the right atrium and, thus, may protect the prosthesis if sternal reentry is necessary. Paraplegia is rarely encountered when using the extraanatomic coarctation bypass technique. A side-biting aortic clamp allows continuation of blood flow to the posterior wall of the aorta. This allows continuation of blood flow to the intercostal arteries and, in turn, reduces the risk of paraplegia. We have encountered no serious complications with this technique; importantly, there have been no episodes of spinal cord ischemia. Cardiopulmonary bypass was used in all patients in this series because of the presence of additional cardiovascular disorders or because of difficulty with exposure of the descending aorta. The ascending aorta was used for arterial cannulation in all but 1 patient included in this series in an effort to optimize cerebral blood during manipulation and clamping of the descending thoracic aorta. In 1996, Pethig et al24 reported hemodynamic instability in 2 patients early after aortic valve replacement and extraanatomic bypass of aortic coarctation. This complication was believed to be due to myocardial ischemia related to low diastolic perfusion pressure in severely hypertrophied hearts. We have not observed this phenomenon in our series of patients. Alternative treatment options include the 2-stage repair of adult coarctation associated with valve or coronary disease20 or surgical intervention of valve disease associated with catheter-based coarctation intervention.15 There are few long-term reports of bypass grafting for coarctation or recoarctation. Potential long-term complications include graft narrowing with thrombus and neointimal formation. Polytetrafluoroethylene ringed grafts are used for this procedure by some groups in an effort to reduce the potential complications of graft kinking and narrowing. Additional potential late complications include infection, development of false aneurysms, and anastomotic dehiscence with pseudoaneurysm formation in patients who have considerable somatic growth after repair. None of the patients that we have reported required reoperation for graft-related complications at a mean follow-up of 3.7 years. We have been hesitant to use this method in preadolescent children because of the risk of anastomotic dehiscence related to somatic growth.12 The surgical management of patients with complex coarctation or recoarctation with or without associated cardiac disorders must be individualized. Extra-anatomic coarctation bypass appears to be a safe flexible method that is particularly useful in adult patients when simultaneous intracardiac repair is required. References 1. Maron B, Humphries J, Rowe R, et al. Prognosis of surgically corrected coarctation of the aorta: a 20-year postoperative appraisal. Circulation. 1973;47:119 –126. 2. Swan L, Wilson N, Houston A, et al. The long-term management of the patient with an aortic coarctation repair. Eur Heart J. 1998;19:382–386. 3. Foster E. Reoperation for aortic coarctation. Ann Thorac Surg. 1984;38: 81– 89. 4. Sweeney M, Walker W, Duncan J, et al. Reoperation for aortic coarctation: techniques, results, and indications for various approaches. Ann Thorac Surg. 1985;40:46 – 49. 5. Cohen M, Fuster V, Steele P, et al. Coarctation of the aorta: long-term follow-up and prediction of outcome after surgical correction. Circulation. 1989;80:840 – 845. 6. Edie R, Janani J, Attai L, et al. Bypass grafts for recurrent or complex coarctation of the aorta. Ann Thorac Surg. 1975;20:558 –566. 7. Wukasch D, Cooley D, Sandiford F, et al. Ascending aorta-abdominal aorta bypass: indications, technique, and report of 12 patients. Ann Thorac Surg. 1977;23:442– 448. 8. Robicsek F, Hess P, Vajtai P. Ascending-distal abdominal aorta bypass for treatment of hypoplastic aortic arch and atypical coarctation in the adult. Ann Thorac Surg. 1984;37:261–263. Connolly et al 9. Heinemann M, Ziemer G, Wahlers T, et al. Extraanatomic thoracic aortic bypass grafts: indications, techniques, and results. Eur J Cardiothorac Surg. 1997;11:169 –175. 10. Vijayanagar R, Natarajan P, Eckstein P, et al. Aortic valvular insufficiency and postductal aortic coarctation in the adult: combined surgical management through median sternotomy: a new surgical approach. J Thorac Cardiovasc Surg. 1980;79:266 –268. 11. Morris R, Samuels L, Brockman S. Total simultaneous repair of coarctation and intracardiac pathology in adult patients. Ann Thorac Surg. 1998;65:1698 –1702. 12. Kanter K, Erez E, Williams W, et al. Extra-anatomic aortic bypass via sternotomy for complex aortic arch stenosis in children. J Thorac Cardiovasc Surg. 2000;120:885– 890. 13. Beekman R, Rocchini A, Behrendt D, et al. Reoperation for coarctation of the aorta. Am J Cardiol. 1981;48:1108 –1114. 14. Grinda J, Mace L, Dervanian P, et al. Bypass graft for complex forms of isthmic aortic coarctation in adults. Ann Thorac Surg. 1995;60: 1299 –1302. 15. Weber H, Cyran S. Initial results and clinical follow-up after balloon angioplasty for native coarctation. Am J Cardiol. 1999;84:113–116. 16. Jacob T, Cobanoglu A, Starr A. Late results of ascending aortadescending aorta bypass grafts for recurrent coarctation of aorta. J Thorac Cardiovasc Surg. 1988;95:782–787. Coarctation of the Aorta I-137 17. Barron DJ, Lamb RK, Ogilvie BC, et al. Technique for extraanatomic bypass in complex aortic coarctation. Ann Thorac Surg. 1996;61: 241–244. 18. Thomka I, Szedo F, Arvay A. Repair of coarctation of the aorta in adults with simultaneous aortic valve replacement and coronary artery bypass grafting. Thorac Cardiovasc Surg. 1997;45:93–96. 19. Hehrlein F, Schlepper M, Scheld H, et al. Combined therapy of re-coarctation of the aorta and coronary heart disease. Thorac Cardiovasc Surg. 1985;33:111–112. 20. Mulay A, Ashraf S, Watterson K. Two-stage repair of adult coarctation of the aorta with congenital valvular lesions. Ann Thorac Surg. 1997;64: 1309 –1311. 21. Folliguet T, Mace L, Dervanian P, et al. Surgical treatment of diffuse supravalvular aortic stenosis. Ann Thorac Surg. 1996;61:1251–1253. 22. Powell W, Adams P, Cooley D. Repair of coarctation of the aorta with intracardiac repair. Tex Heart Inst J. 1983;10:409 – 413. 23. Izhar U, Schaff H, Mullany C, et al. Posterior pericardial approach for ascending aorta-to-descending aorta bypass through a median sternotomy. Ann Thorac Surg. 2000;70:31–37. 24. Pethig K, Wahlers T, Tager S, et al. Perioperative complications in combined aortic valve replacement and extraanatomic ascendingdescending bypass. Ann Thorac Surg. 1996;61:1724 –1726. Downloaded from http://circ.ahajournals.org/ by guest on June 12, 2017 Posterior Pericardial Ascending-to-Descending Aortic Bypass: An Alternative Surgical Approach for Complex Coarctation of the Aorta Heidi M. Connolly, Hartzell V. Schaff, Uzi Izhar, Joseph A. Dearani, Carole A. Warnes and Thomas A. Orszulak Downloaded from http://circ.ahajournals.org/ by guest on June 12, 2017 Circulation. 2001;104:I-133-I-137 doi: 10.1161/hc37t1.094897 Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2001 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/104/suppl_1/I-133 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/