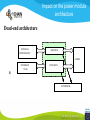
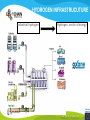
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download FUEL CELL
Survey
Document related concepts
Transcript
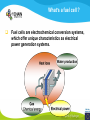
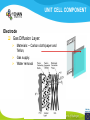
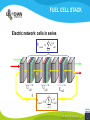
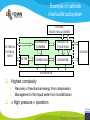
Fuel cell: from principle to application to the electric vehicle Yann BULTEL, GINP Marian Chatenet, GINP Laurent Antoni, CEA Jean-Paul Yonnet, CNRS PLAN 1. 2. 3. 4. 5. 6. 7. Fuel Cell Introduction Fuel Cell – Principle of Operation Fuel Cell Performances Fuel Cell Power System Power Fuel Cell Module Hybridizing Safety issues Fuel Cell Vehicle Example 1. Fuel Cell introduction Yann Bultel What’s a fuel cell ? Fuel cells are electrochemical conversion systems, which offer unique characteristics as electrical power generation systems. What’s a fuel cell ? H2 Heat loss H2Oout, O ANODE H2in 2 / Air CATHODE O2in / Air Electrical power HISTRORICAL CONSIDERATION 1842: Sir William Robert Grove is known as “Father of the Fuel Cell.” reaction triple contact electrolyte-reactants-catalyst HISTRORICAL CONSIDERATION 1932: F. Bacon, Fuel Cell with an alkaline electrolyte AFC Years 50-60 : spatial programs (NASA) First practical use Gemini : 1 kW PEFC (General Electric) Apollo : ~10 kW AFC (Pratt & Whitney) Years 2000 : CEA Fuel Cell Vehicle FUEL CELL TYPES Fuel Cell types: Low temperature Fuel Cells: PEMFC: Proton Exchange Membrane Fuel Cell (ambient to 80°C); PAFC: Phosphoric Acid Fuel Cell (PAFC) (200-250°C); AFC: Alkaline Fuel Cell (ambient to 80°C). High temperature Fuel Cells: MCFC: Molten Carbonate Fuel Cell (600 to 700°C); SOFC: Solid Oxide Fuel Cell (800 to 1000°C). Fuel Cell technology for HYCHAIN vehicles is based on the PEMFC. 2. Fuel Cell – Principle of Operation Yann Bultel and Marian Chatenet UNIT CELL BEHAVIOUR Unit cells form the core of a fuel cell (case of PEMFC): This device converts the chemical energy contained in a fuel electrochemically into electrical energy: Hydrogen oxidation at the anode Oxygen reduction at the cathode Unit Cell working behaviour UNIT CELL BEHAVIOUR Electrochemical Reactions (case of PEMFC): Hydrogen Oxidation: 2 H2 (Dihydrogen)→ 4 H+ (proton) + 4 e- (electron) Oxygen Reduction: O2 (oxygen) + 4H+ (proton) + 4 e- (electron ) → 2 H2O (Water) Whole Reaction: 2 H2 (Dihydrogen) + O2 (oxygen) → 2 H2O (Water) UNIT CELL COMPONENT Unit cell is made of (case of PEMFC): Electrolyte: polymer membrane (Nafion) Gas Diffusion Electrode Gas Diffusion Layer • Carbon cloth/paper Active Layer Carbon + Platinum Unit cell components UNIT CELL COMPONENT Electrolyte Materials: ~ Polymer electrolyte; Example: Nafion perfluorosulfonic acid PTFE copolymer (DUPONT) N-115/117 (130/180 µm) Properties: Protons migrations from anode to cathode; Gas separator; Electronic insulator. UNIT CELL COMPONENT Electrolyte Nafion Non F-ionomers H N N ]n [ N H N UNIT CELL COMPONENT Electrode Active(/Catalyst) Layer: Materials: ~ Carbon grains supported Platinum nanoparticles; Electrochemical reactions (Hydrogen oxidation and Oxygen reduction). UNIT CELL COMPONENT Electrode Active(/Catalyst) Layer: Which electrocatalysts for the cathode ? • Issues O2 reduction slow and not reversible • 4 e- reaction non quantitative (peroxides formation) • high ORR overpotential high catalysts loadings required high cost catalyst utilization ? Instability of the Pt/C particles • Solutions Alloy or composites nanoparticles to improve the 4 e- pathway Pt-Co/C, Pt-Ni/C Non-platinum electrocatalysts? UNIT CELL COMPONENT Electrode Gas Diffusion Layer: Materials: ~ Carbon cloth/paper and Teflon; Gas supply. Water removal UNIT CELL COMPONENT MEA AL GDL Electrocatalyst Electrolyte Carbon Hydrophobicporosity-binding agent (PTFE) Substrate (C fabric) Carbon (powder) Hydrophobicporosity-binding agent (PTFE) Membrane Ionic conducting polymer Electronic insulator Barrier to reagent UNIT CELL COMPONENT MEA Gas Diffusion Layer (GDL) Active Layer (AL) Reagent feeding Products draining (water & reagent excess) Current collecting Thermal management Mechanical support Membrane A & C reagent separation Ionic transport Electronic insulator Mechanical support Electrochemical reactions + function of the GDL 150 µm UNIT CELL COMPONENT External current collecting End plate Electrode Membrane Assembly Bipolar plate UNIT CELL COMPONENT Bipolar plate: Materials Graphite Metallic Properties Electronic current collecting; Gas distribution; Heat management. UNIT CELL COMPONENT Serpentine flow field design: Conventional (a) and interdigitated (b) gas distributor of PEMFC bipolar plate UNIT CELL COMPONENT 1. Serpentine flow field design: l w d Parameter Values channel width w 0.5-2.5 mm channel depth d 0.2-2.5 mm landing width l 0.2-2.5 mm draft angle 0-15° UNIT CELL COMPONENT Single Cell Gas supply Exhaust Gas FUEL CELL STACK The stacking involves connecting multiple unit cells in series via Bipolar plate to provide: An electrical series connection between adjacent cells; Gas barrier that separates the fuel and oxidant of adjacent cells; Fuel Cell Stack description Fuel Cell Stack Principle FUEL CELL STACK Electric network: cells in series n k U stack U cell k 1 e- Ucell Ucell I Ucell n k Pstack I U cell k 1 FUEL CELL STACK Gas supply: cells in parallel ANODE H2in CATHODE O2in / Air 3. Fuel Cell Performances Yann Bultel FUEL CELL PERFORMANCES Actual Cell Potential: Activation-related losses (due to kinetics); Ohmic losses (due electrical resistance); Mass-transport-related losses (due to diffusion losses); FUEL CELL PERFORMANCES The triple contact Gas diffusion e- transfer resistance (activation) H2 Ionic resistance H+ Polarisation curve Er Ei=0 ORR irreversibility 1 Cell voltage U (V) 2 e- 3 Ohmic resistance 1 - Activation overvoltage hact Electrocatalysts, Sact Current density i (A/cm2) 2 – Ohmic drop hohm Ions and e- resistance (membrane, electrodes) 3 – Diffusion limitation hdif Gas diffusion to the catalyst FUEL CELL PERFORMANCES Activation overpotential: Kinetic Tafel Law Overpotential hact Current density i ln 2.3 i0 b Overvoltage at the surface of an electrode Experimental parameters h (V) b : Tafel constant i0 : exchange current density Log(i) FUEL CELL PERFORMANCES Activation overpotential: Kinetic Tafel Law b : Tafel slope Impedance study of the oxygen reduction reaction on platinum nanoparticles in alkaline media, L. Genies, Y. Bultel, R. Faure, R. Durand, Electrochimica Acta (2003) FUEL CELL PERFORMANCES Internal Resistive losses Electronic and Ionic conductivity of materials Nafion: ionic = 5 S m-1 Bipolar plate: ionic = 5000 S.cm-1 Resistance of the flow of ions >> Resistance of the flow of electrons H+ Lm Vionic j R electrolyte j m FUEL CELL PERFORMANCES Mass Transport limitation: finite mass transport rates limit the supply of fresh reactant; O2 Faraday’s law: CB nFD CB CS i Limiting current density jL nFD CB 0 iL Concentration overpotential: hconc CS AL H2O GDL RT i ln1 nF iL FUEL CELL PERFORMANCES Power density versus current density: 1,2 1 0,8 Y/- U/V 0,6 0,4 P/ W cm-2 We I U cell 0,2 0 0 0,2 0,4 0,6 0,8 -2 i / A cm 1 1,2 1,4 FUEL CELL PERFORMANCES Heat production: Electrical power I U W 2 Q R I ohmic Q act conc h I T S Q reversibility .I nF Heating rate H r Q I U heat cell 2F e H2 1 O2H2O 2 Hr Maximum efficiency possible cell FUEL CELL PERFORMANCES Gas feeding: Mass transport into a cell Gas Utilization: Gas consumption (H2, O2) and water production is linked to current the current (/density) Faraday’s law Fi,react I nF [mol.s-1] Ni,react i nF [mol.s-1.m-2] FUEL CELL PERFORMANCES Mass and Heat Balances: PEMFC Stack Mass Balance: Fi,in Fi,out Ncell I nF Heat Balance: i c pi Tout Tin Qcool m FUEL CELL PERFORMANCES One species mass balance O2 or H2: Fi,in Fi,out Fi,react I Fi,out I nF Gas Utilization Rate: Fi,react Fi,in Fi,out Ui Fi,in Fi,in Stoichiometric ratio Fa,in anode Fi,in 1 St i Fi,react Ui Fc,in Sti=1 : Stoichiometric conditions cathode Fa,out Fc,out FUEL CELL PERFORMANCES Water management: Rate of evap/cond Psat PH2O H2O, O2, N2 Gas channel NHelectro 2O 2 drag 2F Cathode H2O i Electro-osmotic flow Anode H2O, H2 Gas channel diffusion H2 O N D H2 O c Ha 2O c Hc 2O Lm H2O Water diffusion electro H2 O r i 2F 4. Fuel Cell Power System Laurent Antoni FUNCTIONAL DECOMPOSITION OF THE FUEL CELL POWER MODULE System analysis: - Numerous possible technical solutions Depends on the application Depends on the environment On board Stationary Portable Temperature Pressure Pollutants… Depends on the user need General objective (power, durability) Duty cycle Analysis / Functional Decomposition FUNCTIONAL DECOMPOSITION OF THE FUEL CELL POWER MODULE FUNCTIONAL DECOMPOSITION OF THE FUEL CELL POWER MODULE AUXILIAIRIES BATTERIES BUS FUEL TANK CODITIONNING INLET/OUTLET CATHODE FUEL CELL CODITIONNING INLET/OUTLET ANODE COOLING : WATER MANAGEMENT SUPERVISOR EXTERNAL ENVIRONNMENT VEHICLE COOLING EXTERNAL ENVIRONNMENT ELECTRICAL CONVERTORS Example of cathode inlet/outlet subsystem COOLING / WATER MANAGEMENT EXTERNAL ENVIRONMENT EXPANSION TURBINE SEPARATOR / CONDENSER CATHODE FILTER COMPRESSOR HUMDIFIER SUPERVISOR Highest complexity Recovery of mechanical energy from compression Management of the liquid water from humidification « High pressure » operation Anode inlet/outlet Influence of the fuel choice On the FC system architecture On-board molecular hydrogen « dead-end » architecture Recirculation circuit Hydrocarbon reforming Steam Reforming or SR, which is endothermic and leads to the best efficiencies but consumes water) Partial Oxidation or POX, which is exothermic and is appropriate for heavy hydrocarbons, AutoThermal Reforming or ATR, a combination of both, facilitating the thermal management of the steam-reforming unit Impact on the power module architecture Recirculation architecture SUPERVISOR EXTERNAL ENVIRONNMENT PURGE Draining RECIRCULATOR CONDENSEUR CONDENSER ANODE A) HYDROGEN TANK DETENDEUR EXPANSION HUMIDIFICATOR HUMIDIFIER COOLING/HUMIDIFICATION Impact on the power module architecture Dead-end architecture EXTERNAL PURGE DRAINING ENVIRONNMENT ANODE HYDROGEN DETENTE EXPANSION TANK B) SUPERVISOR Impact on the power module architecture Steam-reforming of methanol/methane architecture EXTERNAL ENVIRONNMENT C) BURNER ANODE METHANOL TANK VAPORISOR STEAMREFORMER SELECTIVE OXIDATION WATER TANK SUPERVISOR The complexity of the system is strongly increased in the case of the reforming Impact on the power module architecture Fuel architecture: Fuel Storage The volume and weight of each of these systems is compared to gasoline, methanol and battery storage systems (each con-taining (1 044500 kJ) of stored energy Impact on the power module architecture Fuel architecture: CO2 emission Performance and efficiency Efficiency of the energy conversion Efficiency is defined as the relationship between the “product” of the action to evaluate and a reference which should be defined. For the fuel cell, as a converter of chemical energy in electrical energy, the product is in general the provided electric power Power provided (systemoutlet) Efficiency Power injected (systemeinlet) Performance and efficiency Expression of the energetic efficiency • The efficiency of the energy conversion in the stack compared to the HHV of fuel is written: Ustack Istack henergy Ncomb ,e HHHV • If Stcomb is the ratio between the entering fuel flow and that consumed for the production of the usable current I St comb h energy Ncomb ,e 2F n cell Ipile Upile 1 1 1 Ucell n cell St comb HHHV St comb UHHV 2 F H • where UHHHV is a symbolic “voltage” corresponding to the total energy conversion of combustion into electrical energy what cannot be done. This symbolic voltage is usually called thermo neutral voltage Performance and efficiency Expression of the energetic efficiency The power delivered by the system never equals that of the stack as many components consume part of the energy produced by the stack Air compressor Pumps (cooling, recirculation H2) Actuators (valves, pressure regulators) Sensors (pressure, temperature, flow) Supervisor Power electronics Practical efficiency of a system is : h energy hconvert . Ustack Istack Uaux Iaux Ncomb ,e HHHV Performance and efficiency Expression of the energetic efficiency The practical system efficiency expression is: 70 Pile à combustible seule Rendement (%) 60 50 Groupe électrogène 40 30 20 h energy hconvert . Ustack Istack Uaux Iaux Ncomb ,e HHHV 10 0 0 10 20 30 Puissance nette (kW) 40 50 5. Power Fuel Cell module Hybridizing Jean-Paul Yonnet VEHICLE POWER Different order of power of vehicles: Tarins: 4 to 6 MW (Megawatts), fast train like TGV: 6 to 8 MW, Trucks and Buses : 200 to 600 kW (kilowatts), On-road cars: 50 to 100 kW, City cars: 20 to 30 kW, Small vehicles: go-kart, scooters, etc: 0.5 to 5 kW. Energy Flux: Hybrid vehicle have two types of energy source: • A temporary electrical energy storage (Batteries or Supercapacitors), • A second energy source: Internal Combustion Engine (ICE) or Fuel Cell (FC). Power FC module hybridizing Several objectives To reduce the Fuel Cell (FC) stack power, To manage the high power peak transients, To recover the braking energy, To increase the efficiency at low power. Different hybridizing levels depending on the transient electric storage capacity Some % of the FC system power, • Use of batteries for e.g. starting. Up to 50% of the FC system power, • The batteries manages the power peak demands. Higher then 50% of the FC system power, • The FC is mainly used to supply the average energy consumption. The FC system definition will strongly depends on the global architecture HYBRID VEHICLE Hybrid operation: One solution is to make a mechanical coupling of the axis of an ICE (Internal Combustion Engine) and an Electric Motor. It is called the Parallel Hybrid, or Mechanical Transmission Hybrid. Parallel Hybrid Vehicle Advantages and disadvantages of Parallel Hybrids: • Electric machine and the associated converter are dimensioned only for the electric power, • The rotation speed is given by the wheel speed, • Lower cost of the electric parts. HYBRID VEHICLE Hybrid operation: When the additional power source is a Fuel Cell, it cannot create mechanical power. It can supply only electric power. It is why only Series Hybrids are possible with Fuel Cell. Series Hybrid Vehicle Advantages and disadvantages of Series Hybrid: • More simple mechanical structure, • The additional power source can be used at its optimal operation point, • But the converter must be designed for the maximum power. FUEL CELL VEHICLE In the power part of a Fuel Cell vehicle, you have : An Electrical Network at medium or high voltage (80V to 500V), One or several Electrical Machines for the wheel propulsion, Power Electronics to make all the energy conversions, Batteries (or other type of electric energy storage), the Fuel Cell, the Hydrogen storage. Operation of a FC Vehicle 6. Safety issues Yann Bultel HYDROGEN SAFETY 1. 2. 3. 4. 5. 6. Hydrogen is odourless, colourless, tasteless and nontoxic. Hydrogen has a very wide range of flammability. Hydrogen is very buoyant and diffuses rapidly in air. Hydrogen has very low ignition energy. Hydrogen burns with a pale blue, nearly invisible, flame. Hydrogen is non-toxic and non-poisonous. Hydrogen Methane Propane Gasoline Lower flammability limits in air (%) 4 4.4 1.7 1.1 Upper flammability limits in air (%) 75 17 10.9 6.7 0.017 0.290 0.240 0.240 Minimum ignition energy (mJ) HYDROGEN SAFETY Hydrogen flame: 7. Fuel Cell Vehicle Example Yann Bultel HYDROGEN INFRASTRUCUTURE Industrial hydrogen Hydrogen, vector of energy HYDROGEN INFRASTRUCUTURE Goal : An appropriate Hydrogen infrastructure HYCHAIN VEHICLES Hydrogen storage PAC HYCHAIN VEHICLES HYCHAIN VEHICLES Application to vehicle Application to wheelchair Hychain Mini-trans Emscher -Lippe GERMANY Rhône-Alpes FRANCE Castilla León SPAIN Emilia Romagna ITALY