EFFECTS OF NANO-SECOND PULSED ELECTRIC FIELDS (nsPEF) ON HUMAN PROSTATE CANCER
... Electromagnetic fields (EMF) appear in a vast set of frequencies (spectra) that are divided in frequency zones, according to the manner they are produced or used. Frequency plays an important part in measuring non-ionized electromagnetic radiation (radiation, which cannot produce ionization of atoms ...
... Electromagnetic fields (EMF) appear in a vast set of frequencies (spectra) that are divided in frequency zones, according to the manner they are produced or used. Frequency plays an important part in measuring non-ionized electromagnetic radiation (radiation, which cannot produce ionization of atoms ...
Hybrid Anion and Proton Exchange Membrane Fuel
... A novel hybrid fuel cell is described using at least one electrode operating at high pH in an effort to use the high conductivity of Nafion and exploit the electrochemical advantages of high-pH operation. The electrochemical behavior of a hybrid anion exchange membrane (AEM)/proton exchange membrane ...
... A novel hybrid fuel cell is described using at least one electrode operating at high pH in an effort to use the high conductivity of Nafion and exploit the electrochemical advantages of high-pH operation. The electrochemical behavior of a hybrid anion exchange membrane (AEM)/proton exchange membrane ...
Accurate identification of single-nucleotide
... reported for nonamplified single-cell clones obtained through organoid formation (609 per organoid genome)12. The somatic SNVs identified with SCMDA had a spectrum very similar to those of the clones, with CG→TA mutations comprising only 21.3% of all somatic SNVs (Fig. 3b), and the same spectra were ...
... reported for nonamplified single-cell clones obtained through organoid formation (609 per organoid genome)12. The somatic SNVs identified with SCMDA had a spectrum very similar to those of the clones, with CG→TA mutations comprising only 21.3% of all somatic SNVs (Fig. 3b), and the same spectra were ...
E - York University
... an electric current and do work. (batteries, fuel-cells) C An electric current can be used to force non-spontaneous chemical reactions to occur. (electrolysis) C Reactions can be made to occur in a specific place. (electroplating, electropolishing) C The voltage produced by a reaction can be used as ...
... an electric current and do work. (batteries, fuel-cells) C An electric current can be used to force non-spontaneous chemical reactions to occur. (electrolysis) C Reactions can be made to occur in a specific place. (electroplating, electropolishing) C The voltage produced by a reaction can be used as ...
Compartmental Genomics in Living Cells Revealed by Single
... several decades ago when patch-clamp approaches were used to isolate RNA from neurons to profile the gene expression of single cells2 linking genetics to neuronal function3. This approach provided only a snapshot of single cell molecular biology, because once the neuron was impaled by the micropipet ...
... several decades ago when patch-clamp approaches were used to isolate RNA from neurons to profile the gene expression of single cells2 linking genetics to neuronal function3. This approach provided only a snapshot of single cell molecular biology, because once the neuron was impaled by the micropipet ...
Guide for Cell Biology
... * Using models, photographs, or illustrations of structures such as organic molecules and cell organelles, identify the structure and describe its function or role in life processes. * Identifying differences between prokaryotic & eukaryotic cells. * Analyzing studies used to determine key pieces of ...
... * Using models, photographs, or illustrations of structures such as organic molecules and cell organelles, identify the structure and describe its function or role in life processes. * Identifying differences between prokaryotic & eukaryotic cells. * Analyzing studies used to determine key pieces of ...
APPENDIX Table of content Appendix Supplementary Figures and
... Igfbp2 are expressed in the embryonic small intestine (C-E), whereas Cd44, Smoc2 and Olfm4 were detected in the adult ISCs only (F-H). The y-axis indicates the coverage normalized by library size (reads per million). Appendix Figure S2 - Strategies for labelling and quantification of various cell po ...
... Igfbp2 are expressed in the embryonic small intestine (C-E), whereas Cd44, Smoc2 and Olfm4 were detected in the adult ISCs only (F-H). The y-axis indicates the coverage normalized by library size (reads per million). Appendix Figure S2 - Strategies for labelling and quantification of various cell po ...
FUEL CELL
... create mechanical power. It can supply only electric power. It is why only Series Hybrids are possible with Fuel Cell. ...
... create mechanical power. It can supply only electric power. It is why only Series Hybrids are possible with Fuel Cell. ...
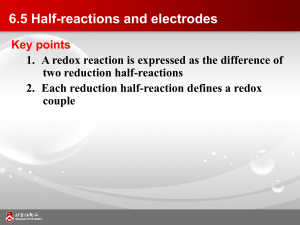
6.5 Half-reactions and electrodes
... 6.5 Half-reactions and electrodes • The electrochemical methods can be used to determine thermodynamic properties of reactions that may be inaccessible by other methods • Electrochemical cell consist of two electrodes (metallic conductors) in contact with an electrolyte (an ionic conductor) • An ...
... 6.5 Half-reactions and electrodes • The electrochemical methods can be used to determine thermodynamic properties of reactions that may be inaccessible by other methods • Electrochemical cell consist of two electrodes (metallic conductors) in contact with an electrolyte (an ionic conductor) • An ...
experiment 7 - (canvas.brown.edu).
... reactions and calculate the standard fuel cell potential, Eo (or open circuit potential), using their standard half cell reduction potentials (See Zumdahl Appendix five, or Tro Appendix II D). ...
... reactions and calculate the standard fuel cell potential, Eo (or open circuit potential), using their standard half cell reduction potentials (See Zumdahl Appendix five, or Tro Appendix II D). ...
General Chemistry 1412
... Electrons are used on the cathode, the reduction of Na+ to Na. Electrons are produced on the anode, the oxidation of Cl-. Electrons are thus flowing from the anode to the cathode. The dc source is forcing electrons to move from the positive to the negative electrode. Remember this is nonspontaneous ...
... Electrons are used on the cathode, the reduction of Na+ to Na. Electrons are produced on the anode, the oxidation of Cl-. Electrons are thus flowing from the anode to the cathode. The dc source is forcing electrons to move from the positive to the negative electrode. Remember this is nonspontaneous ...
irreversible cell
... Advantages : 1. It is a standard reference electrode with which the potentials of other electrodes are calculated 2. The results are highly accurate 3. The error due to electrical leakage is negligible Disadvantages : 1. It requires hydrogen gas and is difficult to set up and transport 2. It require ...
... Advantages : 1. It is a standard reference electrode with which the potentials of other electrodes are calculated 2. The results are highly accurate 3. The error due to electrical leakage is negligible Disadvantages : 1. It requires hydrogen gas and is difficult to set up and transport 2. It require ...
Text S1.
... probed with indicated primary antibodies, washed three times with PBST, incubated with secondary antibody conjugated with horse-radish peroxidase (HRP), and developed using chemiluminescence substrates and X-ray film. For Parkin experiments, cells were harvested and suspended in cell homogenization ...
... probed with indicated primary antibodies, washed three times with PBST, incubated with secondary antibody conjugated with horse-radish peroxidase (HRP), and developed using chemiluminescence substrates and X-ray film. For Parkin experiments, cells were harvested and suspended in cell homogenization ...
Expriment5-labReport-Spring2017
... 3. Calculations: Electroplating (Show all your work.) a. Calculate the number of moles of copper that were plated moles of Cu =_______________ b. Calculate the number of moles of electrons of copper moles of electrons of Cu =_______________ c. Calculate the number of moles of electrons of O2 moles o ...
... 3. Calculations: Electroplating (Show all your work.) a. Calculate the number of moles of copper that were plated moles of Cu =_______________ b. Calculate the number of moles of electrons of copper moles of electrons of Cu =_______________ c. Calculate the number of moles of electrons of O2 moles o ...
Oxidation and Reduction Reactions
... reactivity series for metals and halogens – what do they show? predicting relative strengths of oxidizing and reducing agents from a reactivity series predicting whether or not a reaction occurs based on the reactivity series constructing a reactivity series based on experimental observations of rea ...
... reactivity series for metals and halogens – what do they show? predicting relative strengths of oxidizing and reducing agents from a reactivity series predicting whether or not a reaction occurs based on the reactivity series constructing a reactivity series based on experimental observations of rea ...
1 7 – Electrochemical conversion 1. Introduction Some successive
... their are expensive. And like any container filled with liquid, they can leak. Molten Carbonate fuel cell (MCFC) (fig.4) uses hightemperature compounds of salt (like sodium or magnesium carbonates (chemically, CO3) as the electrolyte. Efficiency ranges from 60 to 80 percent, and operating temperatur ...
... their are expensive. And like any container filled with liquid, they can leak. Molten Carbonate fuel cell (MCFC) (fig.4) uses hightemperature compounds of salt (like sodium or magnesium carbonates (chemically, CO3) as the electrolyte. Efficiency ranges from 60 to 80 percent, and operating temperatur ...
Supplementary Information 1 (doc 48K)
... Cell lines and reagents. HME3 and HMLE3 cells [2] (Obtained from Tan Ince, University of Miami, Miami, FL, USA) were grown in a humidified atmosphere containing 5% CO2 at 37°C in MEGM Mammary Epithelial Cell Growth Medium (Lonza), supplemented with bovine pituitary extract (52 μg/mL), hydrocortisone ...
... Cell lines and reagents. HME3 and HMLE3 cells [2] (Obtained from Tan Ince, University of Miami, Miami, FL, USA) were grown in a humidified atmosphere containing 5% CO2 at 37°C in MEGM Mammary Epithelial Cell Growth Medium (Lonza), supplemented with bovine pituitary extract (52 μg/mL), hydrocortisone ...
Chemistry in Anatomy
... In the body, potential energy is stored as glucose molecule Kinetic energy is when that glucose molecule is used to form ATP, and perform work in the cell ...
... In the body, potential energy is stored as glucose molecule Kinetic energy is when that glucose molecule is used to form ATP, and perform work in the cell ...
Test Objectives for Unit 11: Oxidation/Reduction
... Use Table J to predict whether a particular redox reaction will occur or not. Use a table of standard electrode potentials to determine whether a particular redox reaction will occur or not. Relate chemical activity to oxidizing and reducing strength. Explain the concept of disproportionation. In an ...
... Use Table J to predict whether a particular redox reaction will occur or not. Use a table of standard electrode potentials to determine whether a particular redox reaction will occur or not. Relate chemical activity to oxidizing and reducing strength. Explain the concept of disproportionation. In an ...
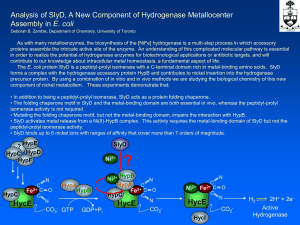
No Slide Title
... As with many metalloenzymes, the biosynthesis of the [NiFe] hydrogenase is a multi-step process in which accessory proteins assemble the intricate active site of the enzyme. An understanding of this complicated molecular pathway is essential in order to realize the potential of hydrogenase enzymes f ...
... As with many metalloenzymes, the biosynthesis of the [NiFe] hydrogenase is a multi-step process in which accessory proteins assemble the intricate active site of the enzyme. An understanding of this complicated molecular pathway is essential in order to realize the potential of hydrogenase enzymes f ...
Electrochemistry of Fuel Cell
... activity is equal to unity. E° is a potential intrinsic to the redox reaction; it is a measure of the ease of ionization of a metal, and of the oxidizing or reducing power of a reaction. The activity ai (i = A, B) is defined as follows. For gases, partial pressure p (atm ; standard condition is 1 at ...
... activity is equal to unity. E° is a potential intrinsic to the redox reaction; it is a measure of the ease of ionization of a metal, and of the oxidizing or reducing power of a reaction. The activity ai (i = A, B) is defined as follows. For gases, partial pressure p (atm ; standard condition is 1 at ...
Galvanic Cell Lab
... Some elements and compounds are more easily reduced than others. They are called strong oxidizing agents. In general, non-metals are more easily reduced than metal ions. Elements that are harder to reduce are easier to oxidize. They are strong reducing agents. Therefore metals are generally easier t ...
... Some elements and compounds are more easily reduced than others. They are called strong oxidizing agents. In general, non-metals are more easily reduced than metal ions. Elements that are harder to reduce are easier to oxidize. They are strong reducing agents. Therefore metals are generally easier t ...
Enzymatic biofuel cell

An Enzymatic biofuel cell is a specific type of fuel cell that uses enzymes as a catalyst to oxidize its fuel, rather than precious metals. Enzymatic biofuel cells, while currently confined to research facilities, are widely prized for the promise they hold in terms of their relatively inexpensive components and fuels, as well as a potential power source for bionic implants.