* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electrostatics Summary
Work (physics) wikipedia , lookup
Elementary particle wikipedia , lookup
Newton's laws of motion wikipedia , lookup
History of subatomic physics wikipedia , lookup
Speed of gravity wikipedia , lookup
Nuclear physics wikipedia , lookup
Electromagnetism wikipedia , lookup
Mass versus weight wikipedia , lookup
Length contraction wikipedia , lookup
Weightlessness wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Atomic nucleus wikipedia , lookup
Fundamental interaction wikipedia , lookup
Lorentz force wikipedia , lookup
Atomic theory wikipedia , lookup
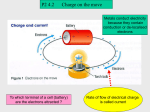
ELECTROSTATICS I. Overview • ELECTROSTATICS is the study of STATIC ELECTRICITY. • Static electricity is an electric charge carried on an insulated object. The object DISCHARGES (transfers) it upon contact with another object. • A static charge can be placed on an object with FRICTION (most common). • To understand static electricity, you need to review the characteristics of the atom. • Static electricity results from a temporary imbalance of the number of protons and electrons in an object. Electrons are gained or lost to create charge, NOT protons. • Opposite charges attract. Like charges repel. This is an important property of objects carrying static electricity. II. The atom and charges The PROTONS (+ charge) and neutrons are in the nucleus The electrons (- charge) surround it. Outermost electrons are “loosely held” and can be made to transfer with friction-rubbing two objects together 1 the atom, continued Materials that hold their electrons tightly are INSULATORS. They resist the flow of electric charge. Materials that hold their electrons loosely are CONDUCTORS. These electrons are willing to “jump” or be displaced toward + charged atoms, including being transferred between objects Atoms on the surface of objects will pick up or lose electrons when two objects are contacted by rubbing. One object loses electrons, the gains them. Both objects have an IMBALANCE of + and – charge. The + charged object has more protons than electrons. The – charged object has more electrons than protons. WE CALL THE CHARGES ATOMS IONS. STATIC ELECTRICITY RESULTS ON BOTH OBJECTS. IT IS THE IMBALANCE OF + AND - CHARGE ON AN OBJECT DUE TO THE GAIN OR LOSS OF ELECTRONS. AN ATOM OR OJECT WITH EQUAL NUMBERS OF PROTONS AND ELECTRONS IS ELECTRICALLY NEUTRAL 2 III. The nature of charged objects A. How charged objects interact OPPOSITE CHARGES ATTRACT LIKE CHARGES REPEL EITHER CHARGE ATTRACTS NEUTRAL OBJECT DUE TO POLARIZATION The ELECTRIC FORCE (a vector) CAUSES THE ATTRACTION OR REPULSION pith ball is polarized when the – charged rod approaches it 3 B. FORCES on a charged particle If the charged object is suspended, then there is a tension force. Gravity is always downward. The repulsive electric field is perpendicular to the gravity force. In this case, the electric field is parallel (opposite direction) to the gravity force. For the paper to lift, the electric force must exceed the gravity force charged rod F electr F grav polarized paper 4 nature of charged objects, continued C. Conducting ability image from the physics classroom on-line hold electrons tightly hold electrons loosely D. How objects can get a static charge When one object obtains electrons from another object, the NET charge is zero. The + charge on the electron poor object is equal in magnitude to the – charge on the electron rich object. 1. FRICTION-One object obtains electrons from another object by rubbing them together. Some materials like to get electrons, some like to give them. The triboelelectric series is a listing of materials in order of their ability to gain electrons. 5 TRIBOELECTRIC SERIES (partial list) + POSITIVE END OF SERIES asbestos glass nylon wool lead silk aluminum paper cotton steel hard rubber nickel & copper brass & silver synthetic rubber orlon saran polyethylene teflon silicone rubber - NEGATIVE END OF SERIES + charged hairs - charged sphere they stand out because they repel each other. 6 2. INDUCTION Remember the concepts of polarization, electronegativity, dipoles van der Waals force, etc from Chemistry? INDUCTION is related to these concepts. neutral molecule but with charge separation (dipole) neutral molecule with even charge distribution (non-polar) + end of the dipole approached non-polar molecule electrons are attracted to the + end of the dipole This phenomenon is why charged objects attract neutral objects Charging by induction works in the same way. INDUCTION INVOLVES PUTTING A CHARGE ON AN OBJECT WITHOUT CONTACT 7 induction, continued - charged rod approaches neutral object induced + charge and – charge form on object as electrons move away from the rod The electrons are “shunted” off of the object by transferring them away (“grounded”) The object now has on it a net + charge A negative charge can be put on the object by using a + charged rod instead. In this case, the ground would give electrons instead of taking them. 8 3. CONDUCTION CHARGING AN OBJECT WITH CONTACT FROM ANOTHER CHARGED OBJECT the rod actually touches the object to be charged charge on the object is the same as the rod this is a thin meal strip. Notice how the ends push away due to repulsion of like charges. This sort of device is called an ELECTROSCOPE 9 IV. COLOUMB’S LAW Coulomb's law states that the electrical force between two charged objects is directly proportional to the product of the quantity of charge on the objects and inversely proportional to the square of the separation distance between the two objects. The mathematical form of Coulomb’s Law ought to remind you of Newton’s Law of Universal Gravitation Q1Q2 F electr = k 2 r Q is the charge, in Coulomb units on each objects r is the distance between the charged objects k is Coulomb’s constant. It’s value depends upon the medium the objects are surrounded by (e.g., air) RECOGNIZE THAT COLULOMB’S LAW IS ANOTHER EXAMPLE WHERE THE STRENGTH OF A FORCE FOLLOWS THE INVERSE SQUARE WITH DISTANCE… IT’S STRENGTH RAPIDLY DIMENISHES WITH DISTANCE 10 V. ELECTRIC FIELD LINES The electric field generated by a particle is a vector. The electric field lines are always drawn pointing AWAY from a + charge. The more arrows that radiate from a particle, the stronger the field being represented. These are drawn by computing the resultant vector after adding the interacting vectors head to tail at a given point in space. This is like the diagram above, lower left but in this case the resultant vectors are shown. The particle on the right is + and the one on left is - 11 V. Strength of the electric field The strength of the electric field of an object is usually determined by placing a + TEST charge in the electric field of the source particle and measuring the force required to move the test charge. This is called the Force/Charge Ratio Combining Coulomb’s Law with the Force/Charge Ratio F electr E= q E is the field strength F is the net force the test charge feels q is the test charge Felectr E= q qQ F electr = k 2 r F electr Q =k 2 q r Q E=k 2 r source charge test charge +Q q r F electr Coulomb’s Law with the test charge as one of the particles This equation is the electric field strength of the particle with charge Q 12