* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Adobe PDF - CL Davis Foundation
Survey
Document related concepts
Transcript
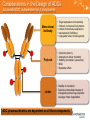
PK/PD and stability of ADCs Sanela Bilic, PharmD, MBA CL Davis Foundation Workshop:“Toxicologic Pathology and Preclinical Development of antibody drug conjugates” Disclaimer: No Novartis data was used in this presentation Thoughts and opinions that will be shared during this talk are based on the available literature as well as my experience gained by working on ADCs 2 The era of ADCs Initiation of brentuximab vedotin Phase III AETHERA Trial, April 2010 Brentuximab vedotin BLA received February 28, 2011 GO voluntary withdrawn from market, June 21, 2010 Initiation of brentuximab vedotin Phase I HL, January 2010 2011 FDA approval of brentuximab vendotin 2000 FDA approval of gemtuzumab ozogamicin (GO) 1980s use of ADCs in clinical trials 1960s first use of ADCs in animal models 1900s Paul Ehrlich introduced the concept of “Magische Kugel”, 3 2013 FDA approval of Kadcyla 2013 Over 20 new ADCs undergoing clinical development (phase I-III) Why ADCs? What's the rationale? Magic bullet story continues Arming antibodies with cytotoxics allows for increased synergy High potential for improved efficacy and lower toxicity Highly complex development: • Regulatory • Manufacturing Insights gained during previous and current ADC development are Concentration aiding in our ability to bring the new ADCs to light Toxicity Efficacy Inefficacy Cytotoxic Toxicity Efficacy Inefficacy ADC John R Adair et al. Antibody-drug conjugates – a perfect synergy. Expert opinion Biol. Ther. 12:9 1191-1206 (2012) Alain Beck et al. The next generation of antibody-drug conjugates comes to age. Discov Med 10:53, 329-339 (2010) Therapeutic index Proof of concept ADC More Efficacious than Free Cytotoxin in Mice MMTV-HER2 Fo5 mammary tumor (HER2-positive, trastuzumab-insensitive) 1500 Vehicle Mean Tumor Volume (mm3 ) +/- SEM DM1 Trastuzumab-mertansine 15 mg/kg, 817 µg/m 2 Trastuzumab 15 mg/kg Trastuzumab 15 mg/kg + Free DM1 817 µg/m2 1000 Free DM1 817 µg/m2 Free DM1 (near MTD) 1947 µg/m2 500 Free DM1 (cytotoxin) T-DM1 (ADC) 0 0 IV Dosing 5 10 15 20 25 30 Day Parsons et al, AACR (2007); Slide adapted from Melissa M. Schutten 2012 presentation Considerations in the Design of ADCs Successful ADC is dependent on 3 components Monoclonal Antibody • • • • • Target expression and selectivity Chimeric, humanized, fully human Limited normal tissue expression Internalization/Trafficking Conjugation sites, Immunogenicity Payload • • • • • Cytotoxic potency Amenable to linker chemistry Stability (circulation, lysosomes) MOA Bystander effect Linker • Stability in circulation • Selective intracellular release of biologically active drug-enzymatic cleavage mAbs degradation ADC pharmacokinetics are dependent on all three components 6 Review of mAb/ADC PK/PD The PK and PD of therapeutic mAbs/ADCs are quite complex, being dependent on both the structure of the antibody and the specific antigen target Being that ADCs are directed against cell-surface antigens, more then likely will exhibit nonlinear PK behavior There are numerous influences that need to be considered: • Receptor shedding, the patient disease state and the physiology of the system being targeted The PD of most mAbs/ADCs appears to be best described using indirect response type models Conducting PD evaluations requires a clear understanding of the interaction of mAb/ADC with the target 7 Small Molecules and Therapeutic Antibodies Small molecules Antibodies ADCs 300-500 Daltons ~ 150,000 Daltons ~ 150,000 Daltons LC/MS/MS ELISA, Gyros, LC/MS/MS ELISA, Gyros, LC/MS/MS Total or free IgG Total IgG, ADC, free toxin, immunogenicity Oral, parenteral Parenteral Parenteral Distribution Vary, binding implies distribution Small, binding implies clearance Small, binding implies clearance Clearance Hepatic/extra-hepatic metabolism Renal/bile elimination Relatively rapid clearance Basal catabolism Receptor-mediated CL IgG level-dependent Renal/bile -uncommon Relatively slow clearance Basal catabolism Receptor-mediated CL IgG level-dependent Renal/bile -uncommon Relatively slow clearance Varies, usually independent of PD 2-compartment behavior Non-linear PK Dependent on PD and physicochemical properties 2-compartment behavior Non-linear PK Dependent on PD and physicochemical properties MW Analytical assay Dosing PK Free or bound drug Small Molecules and Therapeutic Antibodies Pre-clinical PK model PK DDI Purity Impurity Immunogenicity Toxicity Small molecules Antibodies ADCs Multiple species Limited number of species due to limited cross reactivity Limited number of species due to limited cross reactivity Common via enzyme induction/inhibition, etc Not as prominent as small molecules, but there Not as prominent as small molecules, but there. Additional risk due to payload/small molecule component High pure May be heterogeneous May be heterogeneous Well characterized, often related to toxicity Difficult to characterize, Difficult to characterize, may interfere with PK and quantitation, often related to immunogenicity may interfere with PK and quantitation, often related to immunogenicity Generally not immunogenic May be immunogenic May be immunogenic, higher risk if immunogenic Either target-related or non-target-related May be exaggerated, prolong or delayed target related On target and off target toxicity will be exaggerated, prolong or delayed target related Bioanalytical strategy Multiple pharmacokinetic assays Gorovits, Alley, Bilic et al. Antibody–drug conjugate analytes. • (A) Example of a heterogeneous antibody–drug conjugate (ADC) reference standard in vivo and the potential theoretical analytes; • (B) conjugated antibody, where at least one drug is present; • (C) antibody-conjugated drug; • (D) total antibody, includes conjugated and unconjugated antibody; • (E) naked antibody, includes fully deconjugated ADC (i.e., drug-to-antibody ratio 0); • (F) small-molecule catabolites. 10 • The analyte mixtures B–F are defined by gray areas to indicate the parts of the ADC structure that would not be fully measured by the respective assay and colored areas to indicate information that would be fully determined. Pharmacokinetic characterization Cross reactive molecule vs non cross reactive molecule Genentech Kadcyla Data With this simple comparison, Genentech scientists nicely showed the impact of specific target binding and lack of it on pharmacokinetic profile • Dose proportional PK of Kadcyla in rats (no specific binding/no cross reactivity) • Non-linear PK in cyno due to cross reactivity/specific binding 11 Assessment of initial stability of ADCs Assessment of the PK differences observed with comparing total vs ADC PK profile Concentration (ug/mL) Concentration (ug/mL) Total ADC Time (days) Time (days) Higher DAR and impact on stability 12 In B.Bender et al publication, PK of different DAR species was characterized in monkeys The higher conjugated DAR moieties (≥ 3 DM1/trastuzumab) deconjugated faster than lower conjugated DAR moieties B.Bender et al Slower Drug Deconjugation With Uncleavable Linker Concentration (µg/ml) Single IV dose 20 mg/kg ADC Total Ab Uncleavable linker Cleavable linker Days post dose 13 Polson, et al., Cancer Res., 69(6), 2009 Slide adapted from Melissa M. Schutten 2012 presentation More Stable Linker Reduces Systemic Toxicity of ADC in Rats Change in bodyweight (grams) Single IV dose given on Day 1 : CD22-DM1 with cleavable linker Days post dose 14 Polson, et al., Cancer Res., 69(6), 2009 Slide adapted from Melissa M. Schutten 2012 presentation On and off target pharmacodynamics Mechanism of Action On-Target Toxicity Tumor Skin Blood Stream Off-Target Toxicity On-target action 15 (Therapeutic effect) Conclusions ADCs are: • Disease-targeted drug constructs • One of the most emergent and exciting new classes of oncology compounds • Complex drugs with complex characterization - Target antigen, antibody type, linker technology and payload mechanism/potency Reductions in unwanted toxicity are most likely to be achieved by close attention and optimization of linking technologies and stability The observed published ADC efficacy data and the strong pipeline of potentially efficacious new agents is suggesting a very promising path forward 16