* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download elements
Chemical imaging wikipedia , lookup
Transition state theory wikipedia , lookup
Chemical potential wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Degenerate matter wikipedia , lookup
Atomic theory wikipedia , lookup
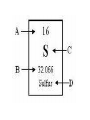
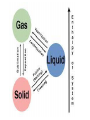
CHAPTER 1 MATTER AND CHANGE *PERIODS – horizontal rows (1-7) *GROUPS – columns (18) a.k.a. families *ELEMENTS – everything is made from ~118 building blocks *SYMBOLS-Shorthand for element names. Composed of 1,2, or 3 letters. THE FIRST LETTER IS ALWAYS A CAPITAL! THE SECOND a/o THIRD LETTER IS ALWAYS LOWERCASE. 3 letter symbols represent temporary names. IUPACInternational Union of Pure and Applied Chemists – In charge of naming elements. There are 11 elements whose symbols are based on archaic names. ELEMENT SYMBOL ORIGINAL NAME LANGUAGE OF ORIGIN Antimony Sb Stibium Latin Copper Cu Cuprum Latin Gold Au Aurum Latin Iron Fe Ferrium Latin Lead Pb Plumbum Latin Mercury Hg Hydrargyrum German Potassium K Kalium Latin Silver Ag Argentum Latin Sodium Na Natrium Latin Tin Sn Stannum Latin Tungsten W Wolfram German *ATOMIC NUMBER – always a whole #. (1-118) -represents the number of protons in one atom (also the # of electrons) *MASS NUMBER(atomic mass, atomic weight) always has a decimal point – round to tenths units are called amu’s The atomic mass is equal to #protons + #neutrons. *Simplest element is #1 – HYDROGEN. (atomic mass=1.00794) *Largest naturally occurring element is #92 – URANIUM. (mass #=238.039) All elements larger than Uranium are man-made or created. *CLASSIFICATION OF ELEMENTS: #1. Majority of elements are METALS. All except Hg are solid @ room temperature. -Properties of METALS: - Luster / Good conductor of heat / Good conductor of electricity / Malleable /Ductile -METALLOIDSsome of the properties of metals (aka semi-metals) Semiconductors are along the stair step line on the right side of chart. -NONMETALSNo metallic propertiescan be solid, liquid, gas. -NOBLE GAS(Inert Gases) The most stable e- arrangement. Normally do not react with other elements to form compounds ALL are gases and are found as single atoms in nature. -The only element that does not fit in these categories is H ! It is an odorless, colorless gas with unique properties, unlike any other element. *ALCHEMY-500-1500AD -Transmutation/Symbols/Secrets *SCIENCEThe study of nature and how it works (natural) *CHEMISTRYBranch of Physical ScienceStudy of matter and the changes it undergoes. *MATTER-Anything that has mass & takes up space (STUFF!) *MASS-Measure of the quantity of matter (how much stuff?) Not the same as weight-does not change . *VOLUME-amount of space an object occupies. *WEIGHT-Measure of the pull of gravity on matter (changes) *DATA (facts)-Measurement of observations during an experiment. (MUST BE A NUMBER AND A UNIT!!!!) *VARIABLE-Data that can change during an experiment. Needs to be kept to a minimum (varies) i.e. X in algebra. The opposite of a “constant” (which doesn’t change) *Model-A replica of a small, large, or distant object or an idea *HYPOTHESIS-1st guess about cause of an observation. (An IF-THEN statement) *THEORY-A widely tested and accepted hypothesis. *LAW- A theory to which no exceptions are known and is thought to be absolutely correct. It does not explain why. *ATOM-The smallest unit of matter. (from ancient Greek word “atomos” for indivisible) The smallest amount of an element that retains ALL of the properties of that element. THE PHYSICAL STATES OF MATTER (PHASES) *SOLID-Definite shape and definite volume. No container. *LIQUID-Definite volume, takes the shape of container. Has the ability to flow from container to container. (no lid needed) *GASEOUS-Has neither definite shape nor volume. Expands to fill the volume of container completely -most compressable. *PLASMA-High temperature, charged particles. A phase change is always physical!! PROPERTIES OF MATTER - PHYSICAL AND CHEMICAL *PHYSICAL-The characteristics and behavior of a substance. Examples include: color, shape, hardness, phase, m.p., b.p., f.p., mass, volume, density, length, width, height… *CHEMICAL-Shows how matter reacts with other substances. Examples include: reactivity, solubility… CHANGES OF MATTER- PHYSICAL AND CHEMICAL *PHYSICALChange that does not cause an altering in the chemical composition of a piece of matter. Examples include: Phase changes, tearing, ripping, shredding, grinding… *CHEMICALInvolve a change in the chemical composition of a substance. Examples: rusting, burning, spoiling, reactions… Indications of a chemical reaction: 1. Formation of a gas. 2. Evolution or heat a/o light. 3. Formation of a precipitate. 4. Color change. *CHEMICAL REACTIONS -Always takes the following form: REACTANTS YIELD PRODUCTS ( means yields) A + B AB ENERGY is ALWAYS involved in Physical or Chemical changes. It can take the form of heat a/o light THE LAW OF CONSERVATION OF ENERGY In a normal chemical reaction, energy is either Absorbed or released. It is not created or destroyed. It only changes form. -During the 1700’s it was discovered that the total mass of the reactants always equaled the total mass of the products! This is now called the LAW OF CONSERVATION OF MATTER -Which states that in a normal chemical reaction, matter cannot be created or destroyed. ELEMENTS, COMPOUNDS, AND MIXTURES *All matter can be classified as: 1)a mixture 2)pure substance. 1. MIXTUREComposed of 2 or more different substances that can be separated by physical means. (filter, distilling, magnets, picking out, centrifuging.) The composition is variable. Mixtures can be classified as: A) homogeneoushas a uniform composition throughout. It has no visible boundaries b/t parts. B)heterogeneoushas a variable composition, boundaries visible 2. PURE SUBSTANCESHas a fixed composition, does not vary from sample to sample. 2 types: A) elements-the simplest pure substances. All samples have the identical physical and chemical properties. It cannot be broken down into a simpler substance and still retain all these properties of the element. B) compounds-pure substances made from the bonding of 2 or more elements.