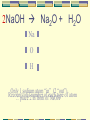* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chemical Reactions
Organic chemistry wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Nuclear fusion wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Stöber process wikipedia , lookup
History of chemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Biochemistry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
History of molecular theory wikipedia , lookup
Hydroformylation wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Marcus theory wikipedia , lookup
Process chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Physical organic chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Rate equation wikipedia , lookup
Click chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Atomic theory wikipedia , lookup
George S. Hammond wikipedia , lookup
Unit 6 – Chemical Reactions Chemical equations, Molar mass, Rates of reactions, Limiting reactants Intro Vocabulary Chemical reaction is the changing of substances to other substances by breaking bonds in reactants and forming new bonds in the products. -when some chemicals come into contact, they break apart, join, or rearrange to form new chemicals (always to become more stable) -produce new substances with new properties Chemical equations are shorthand representations of chemical reactions. Some more vocabulary Reactants are the elements or compounds that enter into a reaction Products are the elements or compounds that are formed as a result of a chemical reaction Arrow () means yields, produces or forms Reactant(s) yield product(s) A + B AB Al2(SO4)3 + Ca(OH)2 Al(OH)3 + CaSO4 Skeleton equation (Unbalanced equation) Consists of symbols and subscripts Symbols: element, yield (), combining (+) Subscript: small number found below the element symbol representing the number of atoms of each element present Al2(SO4)3 + Ca(OH)2 Al(OH)3 + CaSO4 Name the reactants (everything left of arrow) Aluminum sulfate & Calcium hydroxide Types & number of atoms in each reactant Aluminum sulfate Al = 2 S=3 O = 12 Calcium hydroxide Ca = 1 O=2 H=2 Al2(SO4)3 + Ca(OH)2 Al(OH)3 + CaSO4 Name the products (everything right of arrow) Aluminum hydroxide & Calcium sulfate Types & number of atoms in each reactant Aluminum hydroxide Al = 1 O=3 H=3 Calcium sulfate Ca = 1 S=1 O=4 Al2(SO4)3 + Ca(OH)2 Al(OH)3 + CaSO4 Notice that the number of atoms is not the same on both sides of the equation. Al = 2 S = 3 O = 14 Ca = 1 H = 2 On the reactant side Al = 1 S = 1 O = 7 Ca = 1 H = 3 On the product side Balancing equations why & how Why do we balance equations? Law of conservation of mass -atoms are not created or destroyed in an ordinary chemical reaction, just rearranged to form new substances What is used to balance chemical equations? Coefficients -the number before the chemical formula (the number is written normal size – not superscript or subscript) Steps to Balancing Equations 1. Determine the number of atoms of each element in reactants and products 2. Balance by adding coefficients A. Polyatomic ions (if same poly. ion on both sides balance as a chunk) B. Metals C. Nonmetals D. “O” & “H” 3. Recheck your count!!! 2 H2 + O2 2 H2O coefficient subscript H O Only 1 hydrogen 2 oxygen atom “in”“out” (4each “out”) (2 type “in”)of atom Recount total number of place 2 in front of H2O * Only add coefficients, NEVER Δ subscripts Can you ever make just 1 molecule of water? 2NaOH Na2O + H2O Na O H Only 1 sodium atom “in” (2 “out”) Recount total2number type of atom place in frontofofeach NaOH 2 Fe + 3 O2 2 Fe2O3 4 Fe O Recount total atom number each of atom Only 2 1 iron oxygen atom “in”of “in” (2 (4 “out”) (3 type “out”) change place 2 2ininfront frontofofFeFe a4 2Ointo 3 and place 3 in front of O2 Counting molecules/compounds How many molecules of each of the following compounds are present in this equation? Al2(SO4)3 + 3Ca(OH)2 2Al(OH)3 + 3CaSO4 Reactants: Al2(SO4)3 = 1 (when only 1 = no number) Ca(OH)2 = 3 (large 3 in front) Products: Al(OH)3 = 2 (large 2 in front) CaSO4 = 3 (large 3 in front) 2H20 2H2 + O2 How many molecules of oxygen are formed when 2 molecules of water are broken down? one How many molecules of water would be required to form 3 molecules of oxygen? Six The number of molecules does not have to be the same! 2 molecules in, 3 molecules out. Using state symbols When writing chemical equations, the state of each product or reactant may be labeled with the following abbreviations (s) = solid (g) = gas (l) = liquid (aq) = aqueous (solid dissolved in a liquid usually water) NOTE: If the states of matter are not included, you will NOT need to include them. If the states of matter are present, you MUST include them! 7 Diatomic elements 7 elements can not exist as single elements – must exist in pairs if it is JUST that element HONClBrIF These 7 are always H2, O2, N2, Cl2, Br2, I2, F2 Never just write H, O, N, Cl, Br, I, F without being bonded to another element. H20 is okay – WHY? Because O is bonded to another element Steps to using word equation to form formula equations: 1) Write formulas / symbols 2) Check for diatomic elements 3) Add state symbols (if given) 4) Balance (if can’t balance, then recheck formulas!!) Writing chemical equation from word equations Na+1 Br-1 Solid sodium bromide reacts with chlorine gas to yield solid sodium chloride and Na+1 bromine gas. Cl-1 Recheck Balance Check for diatomics (HONClBrIF) Write Add formulas state symbols & element symbols 2 NaBr(s) + Cl 2 (g) 2 NaCl (s) + Na Br Cl Br2 (g) Another word equation Solid aluminum metal reacts with oxygen gas to form solid aluminum oxide. O-2 Al+3 Check for diatomics (HONClBrIF) Write formulas & element symbols Add state symbols Balance Recheck 2 Al (s) + 3 O2 (g) 2 Al2O3 (s) 4 Al O Writing Word Equations Na2O(s) + CO2(g) Na2CO3(s) Solid sodium oxide combines with (reacts with / and) carbon dioxide gas to form (yields/produces) solid sodium carbonate. NaCl(s) + AgNO3(aq) NaNO3(aq) + AgCl(s) Solid sodium chloride and (combines with / reacts with) aqueous silver nitrate forms (yields / produces) aqueous sodium nitrate and solid silver chloride. 5 Basic Types of Reactions Synthesis Reaction Two or more substances combine to form a single substance. Also known as a combination reaction. A + B AB always forming 1 product Example: 2K + Cl2 2KCl 5 Basic Types of Reactions Decomposition Reaction A single compound is broken down into two or more products. AB A + B always having 1 reactant Example: CaCO3 CaO + CO2 5 Basic Types of Reactions Single Replacement Reaction one element replaces another element in a compound (also called single displacement) AB + C AC + B Always a compound + element as reactants Example: Mg + Zn(NO3)2 Mg(NO3)2 + Zn (Mg is Cation so replaces the cation in the compound) 5 Basic Types of Reactions Double Replacement Reaction the positive ions are exchanged between two reacting compounds (also called double displacement) AB + CD AD + CB Always a compound + compound as reactants Example: BaCl2 + K2CO3 BaCO3 + 2KCl (Ba & K are the cation that switch places forming the new compounds) 5 Basic Types of Reactions Combustion Reaction an element or a compound reacts with oxygen often producing energy as heat and light CxHy + O2 CO2 + H2O Always has oxygen as a reactant Is an exothermic reactions (gives off heat) Example: CH4 + 2O2 CO2 + 2H2O Calculating Gram Formula Mass Formula mass can be calculated in amu’s or g’s of a substance by multiplying the number of atoms of each element by the mass in amu’s or g’s of the element. Then add the values together. (YES, sig figs COUNT!!!) Example: CaSO4 (# atoms each element x mass = total mass of element in compound) 1 Ca 1S 4O x x x 40.078g = 40.078 32.066g = 32.066 + + 15.9994g = 63.9976 Then add masses of all elements together 136.1416 = 136.142 The Mole To make a quantity that is measurable in lab, we use the mole. In chemistry one mole is equal to 6.022 x 1023 particles (Avogadro’s number). The gram formula mass of any compound is the mass of 1 mole of the compound in grams. 1 mole = 6.0022 x 1023 is similar to 12 eggs = 1 dozen 52 weeks = 1 year 1 gross = 144 Limiting Reactants The limiting reactant is the reactant that determines the maximum amount of product that is formed. The limiting reactant will be completely used up in a reaction. This makes the reaction stop. The other reactant will have some unchanged so it is said to be the excess reactant. For example, if you need to make 10 chicken sandwiches. You have 10 slices of bread and 10 pieces of chicken. If each sandwich requires 2 slices of bread and 1 piece of chicken, which is the limiting reactant? Excess reactant? Rates of Reactions The reaction rate is the change in concentration of reactants and products in a certain amount of time. Rate at which the reactants disappear and the products appear. Combining two substances (causing a reaction) means forcing their particles to hit, or collide with, one another Collision Theory states that molecules must collide in order to react Activation Energy The activation energy is the energy needed to start the reaction. When particles collide with sufficient energy – at least equal to the activation energy – existing bonds may be disrupted and new bonds can form Endothermic reaction – the energy of the product is greater than that of the reactants (energy is absorbed into the reaction) Exothermic reaction – the energy of the products is lower than that of the reactants (energy is released from the reaction) Factors Affecting Reaction Rates Nature of Reactants Depends on the state of particular reactants and the complexity of the bonds that have to be broken and formed in order for the reaction to proceed The more bonds to be broken then the longer the reaction takes A reaction between two gases will be quicker than a reaction between two liquids or two solids. Factors Affecting Reaction Rates Temperature The higher the temperature at which a reaction occurs, the faster the particles will move The faster the particles move the higher the frequency of collisions For example, food spoils faster at room temperature than when it is refrigerated. Factors Affecting Reaction Rates Concentration Deals with how many particles are there An increase in concentration means that there are more particles within a given volume and thus smaller spaces between the reacting particles. Thus, the higher the concentration of reactants, the greater the frequency of collisions among their particles. For example, the more people there are in a room the more people you will bump into as you walk through the room. Factors Affecting Reaction Rates Surface Area Surface area deals with the number of particles that are exposed for reaction. The larger the surface area the greater the number of particles that are exposed for reaction. For example, many small pieces of coal will burn faster than a lump of coal (small pieces have more particles exposed to react with more oxygen particles) Factors Affecting Reaction Rates Catalysts A catalyst is a substance that increases the rate of the reaction without itself being used up in the reaction (doesn’t appear as a reactant or a product) Catalysts lower the activation energy required for a reaction to occur. Thus a catalyst creates a different pathway from reactants to products – one that requires less energy. Catalysts in the body are enzymes – there to speed up reactions in the body that are essential to life.