* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Note - Science 10
Survey
Document related concepts
Transcript
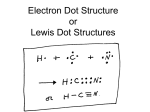
Bohr Diagrams: Bohr diagrams show how many electrons appear in each electron shell around an atom. Each shell holds a maximum number of electrons (2, 8, 8, 18, 18) Electrons in the outermost shell are called valence electrons If the valence shell is full = the atom is stable If the valence shell is not full = the atom is not stable Note: Think of the shells as being 3D like spheres, not 2D like circles! Bohr Diagrams What element is this? • It has 18 protons and it has 2 + 8 + 8 = 18 electrons. 18 p 22 n •It has 8 electrons in the outer (valence) shell Argon! Bohr Diagrams for the first 20 elements Note: The noble gas elements have full electron shells, and are very stable. Bonding Types: When two atoms get close together, their valence electrons interact. Ionic Bonds: Metals give electrons to non-metals (transfer of valence electrons) cations (+ ions) and anions (- ions) form For example, lithium and oxygen form an ionic bond in the compound Li2O + Lithium Oxygen Electrons are transferred from the cations to the anion Li+ O2Li+ Lithium oxide, Li2O Bohr Diagram for LiF - Lithium fluoride On board Covalent Bonds: Formed between two or more non-metals Valence electrons are shared between atoms A group of covalently bonded atoms are called a molecule + Hydrogen Hydrogen fluoride Fluorine Electrons are shared Bohr Diagram for CH4 – Methane (or carbon tetrahydride) On Board Lewis Diagrams: Like simplified Bohr diagrams Only valence electrons are shown Dots representing electrons are placed around the element symbols (on 4 sides, imagine a box around the symbol) Electron dots are placed singularly, until the fifth electron is reached, then they are paired. Example: Nitrogen atom Lewis Diagrams: Note: the Lewis diagrams are the same (except for the symbols) for elements in the same family because they have the same number of valence electrons Lewis Diagrams for Ions: For positive ions: one electron dot is removed from the valence shell for each positive charge of the ion. For negative ions: one electron dot is added to each valence shell for each negative charge of the ion. Square brackets and the charge are placed around each ion Example: Nitrogen ion Lewis Diagrams For Covalent Bonds: valence electrons are drawn to show sharing of electrons. Remember: All atoms “like” to have a full valence shell The shared pairs (“bonding pairs”) of electrons are usually drawn as a straight line “lone pairs” are the electrons not shared • Lewis Diagrams For Ionic Bonds: – • •• • • • • Be • • Cl •• •• • • •• •• Each beryllium has two electrons to transfer away, and each chlorine wants one more electron • • Cl •• •• •• Be •• •• •• •• •• Cl • • • • Since Be2+ wants to donate 2 electrons and each Cl– wants to accept only one, two Cl– ions are necessary • • Cl •• •• • •• Be • • •• 2+ •• • – •• Cl • • •• The ionic compound Beryllium chloride is formed