* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Explaining the Periodic Table (6.7)
Survey
Document related concepts
Transcript
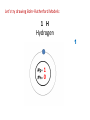
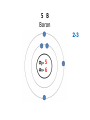
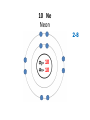
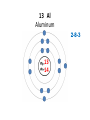
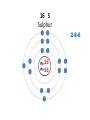
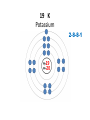
Explaining the Periodic Table (6.7) • If elements are the building blocks of all other matter, what are they made of? • There are three particles that make up an atom or element: • protons • electrons • neutrons • These are called subatomic particles because they are smaller or below an atom. PROTONS are located in the nucleus and have a positive charge. NEUTRONS are also located in the nucleus and have no charge. ELECTRONS are located in orbits around the nucleus. They have a negative charge. Explaining the Periodic Table (6.7) How many ELECTRONS, PROTONS and NEUTRONS are in an atom? atomic number atomic mass (mass number) 6 element symbol C carbon 12.01 element name Atomic Number atomic number • atomic number = # of protons 6 C carbon 12.01 • each element has a unique atomic number • each element is identified by its atomic number and number of protons • (ex. the element carbon, and only carbon, has the atomic number of 6) • In a neutral atom, the number of positives must equal the number of negatives. • This means the # of electrons = # of protons Atomic mass (mass number) 6 atomic mass (mass number) C carbon 12.01 • atoms of an element may have different masses because they have different numbers of neutrons – these are called isotopes • round mass to a whole number mass number = sum of protons + neutrons From the atomic number and mass number of an atom, we can determine the number of protons, electrons, and neutrons in an atom. # protons = atomic number # electrons = # protons (for neutral atoms only) # neutrons = mass number – atomic number Ex) Determine the number of protons, electrons and neutrons in the following atoms: a) Fluorine 9 protons ____ 9 ____ electrons 10 ____ neutrons b) Bromine c) Sodium 35 protons ____ 11 protons ____ 35 electrons ____ ____ electrons 45 ____ neutrons 11 12 ____ neutrons Bohr-Rutherford diagrams are drawings of atoms that show the number of protons and electrons in the nucleus and the number of electrons in each energy level (orbit). Ex) 1st orbit can hold 2 electrons The 2nd and 3rd orbits can hold 8 electrons Let’s try drawing Bohr-Rutherford Models: 1 H Hydrogen 1 0 1 5 B Boron 2-3 5 6 10 Ne Neon 2-8 10 10 13 Al Aluminum 2-8-3 13 14 16 S Sulphur 2-8-6 16 16 19 K Potassium 2-8-8-1 19 20















![Atomic Structure [PowerPoint]](http://s1.studyres.com/store/data/000122096_1-1d100da6540d2f26db122fc51f672fe5-150x150.png)


