* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ANTIBIOTICS
Discovery and development of tubulin inhibitors wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Neuropharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Antibiotics wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
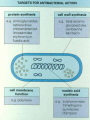
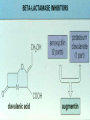
ANTIBIOTICS http://www.tcd.ie/Clinical_Microbiology • • • • Local Home Page A-Z Pick C Go to Clinical Microbiology ANTIBIOTICS • Agents (may be given iv/po/im) used to treat or occasionally prevent infection ( known as Prophylaxis), also known as antimicrobials (includes antibacterial agents, antifungal agents, anti-parasitic agents) • NB: Samples from site of infection to be sent to laboratory for identification and antibiotic susceptibility profile- means appropriate antibiotic may be selected CONTENTS OF LECTURE • Basis of Antimicrobial Action • Site of Action • Examples of Antibiotic Class, Site of Activity, Spectrum of Activity, Toxicity, Resistance to Antibiotic • Antibiotic Policy, Audit, Surveillance • Brief Outline of Antifungal/Antiviral Therapy ANTIBIOTICS • Biologically produced by filamentous fungi and bacteria of the actinomycete family. • The key to their mode of Action is Selective Toxicity: the ability to inhibit bacteria or other microorganisms, without affecting the host adversely- balance of toxicities • Action: may be narrow spectrum or broad spectrum CHOICE OF ANTIBIOTIC • Antibiotics should only be used if indicated • Inappropriate use may be detrimental to the patient e.g.non-treatment of infection, change of normal flora, development of pseudomembranous colitis And detrimental to the wider community encouraging the development of Resistance The House of Lords select committee( Path to Least Resistance) and SARI Report ( A Strategy for the Control of Antimicrobial Resistance in Ireland ) Get Smart campaign in the U.S CHOICE OF ANTIBIOTIC IS DETERMINED BY: Bacteria causing infection in that particular patient, VERY IMPORTANT to SEND SAMPLES FROM INFECTION SITES of patient to Microbiology for gram stain and culture and sensitivity profiles Spectrum of Activity HOST FACTORS: Pharmacokinetics/Pharmacodynamics Presence of Resistance Four things SARI REPORT APRIL 2001 RECOMMENDED STRATEGIES • Recommended Infrastructure • Surveillance of Antimicrobial Resistance • National Reference Laboratories • Monitoring Supply and Use of Antimicrobials • Development of Guidance for Appropriate Use of Antibiotics • Education in relation to appropriate use of Antibiotics • Development of Principles in relation to Infection Control • Future Research in the Area Better Prescribing Complications Appropriate ANTIBIOTICS May be: • Bacteriostatic – inhibits growth of Bacteria, so acts by preventing bacteria from multiplying and then hosts defences deal with the small number of bacteria left • Bactericidial – kills Bacteria, so eliminates bacteria HOW TO KILL A CELL • Damage to CELL WALL or inhibition of it`s synthesis • Damage to cell membrane • Alteration of cell proteins and/or nucleic acids • Enzyme Inhibition • Inhibition of nucleic acid /protein FOUR MAIN TARGETS OF ANTIBIOTICS IN BACTERIA • • • • Cell Wall Synthesis Protein Synthesis Nucleic Acid Synthesis Cell Membrane Function EXAMPLES OF ANTIBIOTICS WORKING AT TARGET SITES • Cell Wall Synthesis: B-Lactams e.g. Penicillins, Cephalosporins and Glycopeptides e.g. Vancomycin • Protein Synthesis: Aminoglycosides, Erythromycin,Tetracyclines, Chloramphenicol, Fusidic Acid • Nucleic Acid Synthesis: Quinolones, Rifampicin • Cell Membrane Function: Polymyxin, Amphotericin 3 MECHANISMS OF RESISTANCE • Alteration in Target Site: target enzyme altered so that it has a lowered affinity for the antibacterial (or additional target enzyme may be synthesized) • Altered Uptake: so decreasing amount of drug reaches the target, may do this by altering entry into the cell or pumping drug out i.e Efflux Mechanism MECHANISMS OF RESISTANCE • Production of enzymes which modify or destroy the antibacterial agent (drug inactivation) –very important e.g. Blactamases, aminogylcoside modifying enzymes • ALL TYPES OF RESISTANCE MAY BE CHROMOSOMAL OR PLASMID OR TRANSPOSON MEDIATED For each Antibiotic consider • • • • Mechanism of Action Spectrum of Activity Toxicity Resistance Cell Wall Synthesis Inhibitors CELL WALL SYNTHESIS INHIBITOR ANTIBIOTICS B-Lactam antibiotics • Structure: large family of compounds all containing the B-Lactam ring. The different members are distinguished by the structure of the ring attached to the B-Lactam ring. • Examples: Penicillins, there is a five member ring attached to the B-Lactam ring, in Cephalosporins a six member ring . Blactam ring Mechanism of Action • Peptidoglycan: a vital component of the cell wall, unique to bacteria. • B-Lactam antibiotics inhibit cell wall synthesis by binding enzymes called Penicillin Binding Proteins which are responsible for the final stages of peptidogylcan (cell wall) crosslinking or formation, subunits acculmulate in the cell Cell Lysis or Death Spectrum of Activity • Benzylpenicillin: mainly active against Gram Positive organisms e.g. Streptococci and Staphylococci • Ureidopenicillins: active against certain gram positive and gram negative organisms • Cephalosporins: Broad spectrum of activity gram negative and positive organisms • Carbapenems : has a broad spectrum of activity even against ESBL producing organisms NEVER GIVE INTRATHECIALLY:B-Lactam antibiotics TOXICITY • Serious Allergy : to B-Lactam drugs in the form of immediate hypersensitivity reaction occurs in ~0.004-0.015% of treatment courses • Mild idiopathic reactions e.g. rash in up to 25% esp. with ampicillin • 10-20% of those allergic crossreact with cephalosporins • In renal impaired patients Benzylpenicillin can produce neurotoxicity in high doses RESISTANCE TO BLACTAM ANTIBIOTICS • 3 Mechanisms • Alteration in target site: MRSA produces an additional PBP, which has lower affinity for BLactams and thus cell wall synthesis continues even when other PBP`s are inhibited: Resistant to all B-Lactam antibiotics • Alteration in access to the target: Gram negative bacteria display this , porin channels alter structure and B-Latam antibiotic cannot get to their target RESISTANCE TO B-LACTAM ANTIBIOTICS • Production of B-Lactamases: these enzymes are produced by the bacteria and catalyse the hydrolysis of the B-Lactam ring , so that antibiotic is inactive, some inactivate some e.g. ampicillin but not others cloxacillin or meropenem To overcome this mechanism of resistance , B-Lactamase inhibitors are added to the antibiotic e.g. clavulanic acid added to ampicillin = augmentin CELL WALL SYNTHESIS INHIBITORS Glycopeptides • Large molecules , e.g vancomycin, teicoplanin • Mechanism of Action: Act at an earlier stage than Blactams , interfers with cell wall synthesis by binding to D-ala-D-ala at the end of the pentapeptide chains, part of growing cell wall Absortion/Excretion • Must be given I.V. for systemic infections as not absorbed from the intestinal tract (useful property when treating C.difficile diarrhoea, when need to treat the organism in the bowel lumen) • Excretion by the Kidneys Spectrum Of Activity • NB: Only active against GRAMPOSITIVE organisms • Used to treat Staphylococci resistant to BLactam antibiotics e.g.MRSA or in patients allergic to B-Lactam drugs TOXICITY • Redman Syndrome: must be given slowly over at least an hour I/V • Potentially Ototoxic • Potentially Nephrotoxic • Important to assay blood levels RESISTANCE • Resistance can occur especially in enterococci i.e. Vancomycin resistant enterococci (VRE)- may be chromosomal or plasmid mediated ANTIBIOTICS THAT INHIBIT PROTEIN SYNTHESIS AMINOGLYCOSIDES Structure: Related molecules containing either strepitidine(streptomycin) or 2deoxystreptamine (e.g. gentamicin) Mechanism of Action: Aminoglycosides kill and inhibit organisms by intering with the binding of formylmethionyl- t RNA to the ribosome, this prevents the formation of initiation complexes from which protein synthesis proceeds. AMINOGLYSOCIDES ABSORPTION/EXCRETION • Cannot be given orally as it is not absorbed from the gut , so must be given I/V OR I/M • They do not penetrate well into tissues, bone or cross the blood brain barrier • They are excreted by the KIDNEYS AMINOGLYSOCIDES-USES • GRAM-NEGATIVE SERIOUS INFECTION esp. SEPTICAEMIA e.g. Pseudomonas at dose 5-7 mg.kg per day once daily dosing • Syneristic activity with BLactam antibiotics against Staphylococci and Streptococci • STREPTINOMYCIN Used for mycobacterial infections and B-Lactam resistant N.gonorrhoeae AMINOGLYSOCIDESTOXICITIES • Potentially NEPHROTOXIC and OTOTOXIC • Small therapeutic range , therefore ANTIBIOTIC ASSAY very important especially if patient at risk or has renal impairment AMINOGLYSOCIDESRESISTANCE 2 Mechanisms Most important is the production of aminoglycoside-modifying enzymes- often plasmid mediated and transferable between bacteria, the aminoglycoside ia altered in structure resulting in change of uptake of antibiotic, this occurs by one-step mutation The 2nd mechanism is alterations in cell permeability of cell wall through porin channels PROTEIN SYNTHESIS INHIBITORS-MACROLIDESetc • Macrolides, Lincosamides, Streptogramins • These group share overlapping sites on ribosomes and resistance to macrolides confers ressitance to the other two groups • Examples: Macrolides-erythromycin and Lincosamides-clindamycin MACROLIDES • STRUCTURE: Large cyclic molecules which all contain a macrocyclic lactone ring • Mechanism of Action: Erythromycin binds to the 23S r RNA in the 50S subunit of the ribosomes and blocks the translocation step in protein synthesis, thereby preventing the release of t RNA after peptide bond formation MACROLIDESABSORBTION/EXCRETION • Can be administer orally and I/V • Well distributed through the body, even for intracellular organisms • Drug is concentrated in th liver and excreted in the bile, some also seen in the urine • Uses: Active against gram-positive organisms , alternative for Penicillin allergic patients, also active against Legionella, Campylobacter species and mycoplasmas, chlamydiae and rickettsiae MACROLIDES-TOXICITY • Relatively non-toxic but may cause nausea and vomiting after oral administration, occasionally causes jaundice MACROLIDES-RESISTANCE • Due to alteration in the 23S r RNA target by methylation of two adenine nucleotides in the RNA. • Resistance is plasmid mediated and inducible ( i.e the presence of erythromycin induces expression of resistance ) INHIBITORS OF NUCLEIC ACID SYNTHESIS • Inhibitors of Nucleic Acid Precursors: Sulphonamides, Trimethoprim • Inhibitors of DNA replication: Quinolones • Inhibitor of RNA polymerase: rifampicin Quinolone Antibiotics • Examples: Nalidixic acid, Ciprofloxacin • Mechanism of Action: Inhibit the activity of DNA gyrase and thereby prevent supercoiling of the bacterial chromosome , this is specific to bacterial cells and thos enot affect mammalian cells Quinolone Antibiotics ABSORPTION etc • Adminstered mostly orally, well absorbed from GI tract • Excreted mostly in the urine • Well distributed throughout the body • • • • • • Spectrum of Activity Gram negative bacteria Urinary tract infections Chlamydiae, Rickettsiae Legionella species Atypical mycobacteria Levofloxacin /Moxifloxacin have broader spectrum against Gram positive organsisms QUINOLONES TOXICITY • Gastrointestinal disturbances most common • Neurotoxicity and Photosensitivity less common 1-2% • In children possible toxic effects on cartilage development • Again may result in normal flora upset QUINOLONE RESISTANCE • Spontaneous Chromosomal Resistance • 2 Forms • Changes in the DNA gyrase subunit structure of the bacteria resulting in lower affinity for the drug • Changes in cell wall permeability- if other drugs enter by this route there may be cross resistance ANTIBIOTIC PROPHYLAXIS • • • • • MICROBIAL FLORA CHOICE OF ANTIBIOTIC TIMING OF ADMINSTRATION DURATION SIDE EFFECTS ANTIBIOTIC PROPHYLAXIS • Patient of normal • Patients undergoing susceptibility exposed certain Surgery e.g. to specific pathogens abdominal surgery e.g. Neisseria • Patients with increased meningitidis causing susceptibility to meningitis. infection e.g. • Patients at risk of Prosthetic heart valves development of TB, or post splenectomy e.g. HIV patients exposed to open TB ANTIBIOTIC ASSAYS • Assay when an antibiotic has a narrow therapeutic index e.g Aminoglysocides • Assay when normal route of excretion iis impaired e.g. patient with renal impairment on vancomycin • Assay in patients receiving prolonged therapy for serious infection e.g. endocarditis • Assay in Neonates with serious infection • Assay if failure to respond to therapy • Assay to check compliance EXAMPLE OF ANTIBIOTIC ASSAYS • Aminoglysocides e.g. gentamicin • Glycopeptides e.g. vancomycin • Isoniazid : to ensure compliance with therapy especially in substance abusers • Aminoglycosides and Glycopeptides : trough level: taken immediately before dose is given and Peak : taken 1 hour post dose • Aminoglycosides: trough correlates with toxicity • Glycopeptides: Trough correlates with adequate therapetic level. ANTIVIRAL THERAPY • • • • Prevention of viral infection Virucides: e.g warts and cryotherapy Antivirals: inhibit viral replication Prevention of viral infections with the use of standard precautions and vaccines e.g. Hepatitis B Immunoglobulin may be given in certain cases to prevent infection such as rabies NEWER ANTIFUNGAL AGENTS • • • • ITRACONAZOLE LIPOSOMAL AMPHOTERICIN VORICONAZOLE DEVELOPING NEW AGENTS: ANTIFUNGAL PEPTIDES SUCH AS ECHINOCANDINS e.g. Capsofungin HAART for HIV infection • Highly Active Antiretroviral Therapy • PROTEASE INHIBITORS e.g Saquinavir • NUCLEOSIDE-REVERSE-TRANSCRIPTASE INHIBITORS e.g ZDV, Didanosine (ddl), 3TC • NON-NUCLEOSIDE REVERSETRANSCRIPTASE INHIBITORS e.g Nevirapine • Recommended antiretroviral: 2 NRTI`s + PI or NNRTI`s