* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download FUNCTIONAL GROUPS ORGANIC COMPOUNDS CONTAINING
Survey
Document related concepts
Transcript
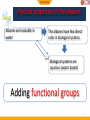
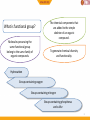
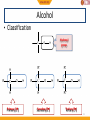
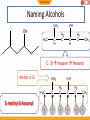
BIO-ORGANIC CHEMISTRY (Organic Chemistry for Biology Students) (SQBS 1603) Organic Compounds Containing Oxygen Dr Nik Ahmad Nizam Bin Nik Malek, BSc (Ind. Chem.)(UTM), MSc (Chem)(UTM), PhD (Chem)(UTM), A.M.I.C Senior Lecturer, Department of Biotechnology and Medical Engineering Faculty of Biosciences and Medical Engineering Physical properties of the alkanes Alkanes are insoluble in water The alkanes have few direct roles in biological systems Biological systems are aqueous (water based) Adding functional groups 2 What is functional group? Molecules possessing the same functional group belong to the same family of organic compounds. The chemical components that are added to the simple skeleton of an organic compound. To generate chemical diversity and functionality. Hydrocarbon Groups containing oxygen Groups containing nitrogen Groups containing phosphorus and sulfur 3 Amines Amides Thiols phosphate functional groups Alcohol Ethers Ketone Aldehyde Esters Carboxylic acids Functional groups Biological importance of functional group Functional group Alcohol Ethers Ketone Aldehyde Carboxylic acids Ester Amines Amides Thiol Phosphate Biological importance Lipids, carbohydrates Archael plasma membranes Metabolic intermediates Reducing sugars such as glucose Lipids, proteins Bacterial and eukaryotic plasma membranes Proteins, nucleic acids DNA and RNA Proteins, nucleic acids DNA and RNA Protein structure, Energy metabolism ATP, DNA 5 Functional groups Groups containing oxygen Chemical class Group Formula Alcohol Hydroxyl ROH Ketone Aldehyde Carbonyl RCOR' Aldehyde RCHO Structural Formula Prefix Suffix hydroxy- -ol keto-, oxo- aldo- -one -al Example Methanol Methyl ethyl ketone (Butanone) Acetaldehyde (Ethanal) Functional groups Groups containing oxygen Chemical class Carboxylate (Carboxylic acid) Group Formula Structural Formula Prefix Suffix Example Acetic acid Carboxyl RCOOH Esters Ester RCOOR' Ethers ether ROR Carboxy- -oic acid Ethyl butyrate alkyl (Ethyl butanoate) alkanoate Di- -ether Diethyl ether 7 Functional groups Groups containing nitrogen Chemical class Group Formula Structural Formula Prefix Suffix Example Dimethylamine Amines Amino RNH2 R2NH R3N amino- -amine Acetamide Amide Amide RCONH2 RCONHR’ RCONR2 Carboxamide- -amide Functional groups Groups containing sulphur and phosporus Chemical class Thiol Group Sulfhydryl Formula RSH Structural Formula Prefix MercaptoSulfanyl- Suffix -thiol Example Ethanathiol (Ethyl mercaptan) Glyceraldehyde 3-phosphate Phosphate phoshate ROP(=O)(OH)2 phospho- 9 Alcohol • Alcohol – Hydroxyl group : -OH functional group – Generic formula: R-OH – General structure R O H Alcohol • Classification C C Hydroxyl group H R' H R O O H Primary (1) H R C R' O H Secndary (2) H R C O H R'' Tertiary (3) 11 Naming Alcohols CH3 OH OH H3C H2 C C H H2 C C H CH3 C : 6 hexane hexanol Methyl at C5 CH3 H3C C H 6 5 OH H2 C C H H2 C CH3 5-methyl-3-hexanol 4 3 2 1 12 Naming Alcohols • Give the IUPAC name of the following alcohol 2 Methyl at C3 H3C OH 3 1 4 6 5 C : 6 cyclohexane cyclohexanol 3-methyl-cyclohexanol 13 Naming Alcohols OH OH HO Ethanol -diol IUPAC name: 1,2-ethanediol or Ethane-1,2-diol Common name: ethylene glycol 14 Reactions of alcohol • Dehydration • Oxidation 15 Dehydration • Loss of water (H2O) • Elimination reaction – Elements of the starting material are lost and a new multiple bond is formed H2SO4 C C H OH C C alkene H2O is lost H2O Dehydration • Examples H H H C C H OH H H2SO4 H H C C H H2O H Ethene Ethanol OH H2SO4 H2O H Cyclohexanol Cyclohexene 17 Dehydration • Zaitsev rule – The major product in elimination is the alkene that has more alkyl groups bonded to it. H H H C C H OH CH2CH3 H2SO4 H CH2CH3 C H H But-1-ene Butan-2-ol H H3C C H C C CH3 OH H Butan-2-ol H2SO4 H3C CH3 C C H H But-2-ene Major product 18 Oxidation • Primary (1) alcohols – Oxidized to aldehydes (RCHO) – And further oxidized to carboxylic acids (RCOOH) O OH [O] [O] R C C H R H H alcohol O aldehyde C R OH Carboxylic acid 19 Oxidation • Secondary (2) alcohols – Oxidized to ketones (R2CO) O OH [O] R C C H R R' R' alcohol ketones 20 Oxidation • Tertiary(3) alcohols – They are not oxidized OH R C R'' R' 21 Physical properties of alcohols H H H H H C C H C C H H H H H C H H C C H H H C C H H H H OH C H H H hydroxyl group oxygen + hydrogen highly electronegative atom polar molecules 22 Physical properties of alcohols hydroxyl group oxygen + hydrogen highly electronegative atom polar molecules hydrogen bonding Strong non-covalent forces Exhibit higher melting and boiling points than alkanes dipolar interaction dispersion forces soluble in water in small quantity 23 Physical properties of alcohols Ethanol 3HC Low electron density H2 C - O Electron pulls towards highly electronegative oxygen atom + H 3HC H2 C high electron density - + O H The distribution of electrons within the molecules is unequal The molecule is polar 24 Physical properties of alcohols hydrogen bonding O H H H2 C H3C H O O H H Ethanol is attracted to the water molecules through hydrogen bonds O H H 25 Ether – Alkoxy group : combination of an alkyl group and oxygen atom (alkyl + oxygen = alkoxy) – Generic formula: R-O-R’ – Similar to alcohol (hydroxyl group), except that the H atom is replaced with an alkyl group O R O H Hydroxyl group R R' Alkoxyl group 26 Ether R O R groups are the same H3CH2C O R H3C CH2CH3 O CH2CH3 R groups are different 27 Naming Ether Simple ethers: Usually assigned common names O Methyl- O Ethyl- Ethyl methyl ether (methoxy-ethane) Ethyl- Ethyl- diethyl ether (ethoxy-ethane) 28 Naming Ether More complex ethers: IUPAC name H3C O Methoxy- H3CH2C O Ethoxy- 29 Naming Ether 6 8 9 7 9 C nonane 5 2 4 3 1 O H3CH2C O Ethoxy- at position 3 3-ethoxy-nonane 30 Physical properties of ethers Presence of oxygen polar molecules O H3C CH3 Two polar bonds 31 Aldehydes and Ketones O Carbonyl group Carbonyl carbon C O O Aldehyde Ketone C R 32 C H R R Aldehydes and Ketones O O Aldehyde Ketone C C R O O C H3C H H Acetaldehyde C R R O O C C H O C H3C CH3 Propan-2-one 33 Naming aldehydes IUPAC name O 4 C butane butanal H CH3 4 H3 C CH 3 O 2 CH C 1 CH3 2,3-dimethylbutanal 34 Methyl at 2 and 3 dimethyl H Naming aldehydes • Simple aldehydes have common names that are widely used • Examples: O O O C H C C H H Formaldehyde 35 H3C H Acetaldehyde Benzaldehyde Naming Ketones IUPAC name O 5 C pentane pentanone C O 1 H3C C 2 3 CH H3C 3-methyl-2-pentanone 36 H2 C 4 CH3 5 Carbonyl position 2 Methyl position 3 Naming Ketones • Common names for ketones: – Naming both alkyl group on the carbonyl carbon. – Arranging them alphabetically. – Finally, adding the word ketone. O O methyl ethyl C H3C CH2CH3 IUPAC name: 2-butanone 37 C H3C CH2CH3 common name: Ethyl methyl ketone Physical properties of aldehydes and ketones Presence of oxygen polar molecules Ether Aldehyde ketone - - O O H3C CH3 C R Two polar bonds Electrons O pulled towards highly C electrone H R gative oxygen atom …….generating an uneven distribution of electron: polar 38 molecule R Reactions of aldehydes and ketones 1. Oxidation of aldehydes O O [O] C R C H R Aldehyde OH Carboxylic acid 2. Reduction of aldehydes and ketones (addition reaction) C 39 O X Y C O X Y Oxidation of aldehydes • Examples O H3CH2CH2C O K2CrO7 C H3CH2CH2C C H OH Butyraldehyde Butyric acid • How about ketones? O H3CH2C K2CrO7 C No Reaction CH3 40 Butan-2-one Reduction of aldehydes and ketones (addition reaction) • Reduction – Decrease in the number of C-O – Increase in the number of C-H bonds • The conversion of a carbonyl group (C=O) to an alcohol is a reduction. – The starting material has more C-O bonds than the product C 41 O X Y C O X Y Reduction of aldehydes and ketones (addition reaction) • Aldehydes – Reduce to primary (1) alcohol H O [H] R C R H Aldehyde H H H [H] H3C C H Acetaldehyde 42 O primary alcohol O H3C C C O H H Ethanol (CH3CH2OH) Reduction of aldehydes and ketones (addition reaction) • Ketones – Reduce to secondary (2) alcohol H O [H] R C R R' ketone R' H Secondary [H] H3C C CH3 Propan-2-one 43 O H O H3C C C O CH3 H Propan-2-ol ((CH3)2CHOH) Acetal formation • Aldehydes and ketones undergo addition reaction with alcohols to form hemiacetals and acetals. R'-OH O R C R H (or R) Aldehyde or ketone H2SO4 O H C OR' H (or R) hemiacetal 1 C is bonded to: -One OH group -One OR group 44 OR'' R''-OH R H2SO4 C OR' H (or R) acetal 1 C is bonded to: -two OR group Acetal formation • Example CH3CH2OH O H2SO4 Propionaldehyde CH3CH2OH H3C H2SO4 CH3 H2SO4 H 1-Ethoxy-propan-1-ol 1,1-Diethoxy-propane hemiacetal acetal O H CH3CH2OH O CH2CH3 Ethanol H3C C OCH2CH3 H3C C OCH2CH3 Propan-2-one 45 H Ethanol C O CH2CH3 H3CH2C C OCH2CH3 H3CH2C C OCH2CH3 H O CH3CH2OH Ethanol Ethanol C H3CH2C O H CH3 2-Ethoxy-propan-2-ol hemiacetal H2SO4 CH3 2,2-Diethoxy-propane acetal Cyclic hemiacetals 1 C is bonded to: -One OH group -One OR group OH OH OH R C OR O H (or R) O 1 C is bonded to: -One OH group -An OR group that is part of a ring 46 Cyclic hemiacetals • Formation of cyclic hemiacetals – Intramolecular reaction of a compound that contain both a hydroxyl group (-OH) and aldehyde or ketone. O O 5 4 3 2 HOH2CH2CH2CH2C C 1 5-Hydroxy-pentanal 47 2 H OH 1 H 2 OH 3 4 5 1 3 4 O 5 Carboxylic Acids • Carboxyl group: Combining the hydroxyl and carbonyl. • Generic formula: RCOOH or RCO2H O carbonyl C R 48 OH hydroxyl Naming Carboxylic acids • IUPAC system suffix –oic acid O 6 C hexane hexanoic acid OH CH3 6 H3 C CH 5 O 4 CH CH3 4,5-dimethylhexanoic acid 49 H2 C 2 3 C H2 C 1 OH 2 methyl at position 4 and 5 4,5-dimethyl- Naming Carboxylic acids • Many simple carboxylic acids are often referred to by their common names. O O O C C C H 50 OH H3C OH OH Formic acid Acetic acid IUPAC name: methanoic acid IUPAC name: ethanoic acid Benzoic acid IUPAC name: benzenecarboxylic acid Physical properties of carboxylic acids 2 oxygen atoms O C R O H 2 very electronegative atoms attach with each other 51 Physical properties of carboxylic acids O • Polarity? C H R O • Dipolar interaction? • Hydrogen bonding between molecules of same compound? • Hydrogen bonding with water? • Solubility in water? • Boiling point? 52 Physical properties of carboxylic acids Presence of oxygen + hydrogen (carbonyl and hydroxyl group) polar molecules O O C R O H O C O O C 53 O H H O C R R R O H Hydrogen bonding between molecules of same compound C R O H Physical properties of carboxylic acids • Hydrogen bonding with water? • Solubility in water? H H H H O O O C R O H H H O 54 The acidity of carboxylic acids O O C R H3O H2O O C H R carboxylic acid carboxylate anion O O H3O H2O C H3C O O Acetic acid H C H3C O Acetic acid anion acetate 55 Reaction with bases O C H3C O H Acetic acid O H-OH NaOH C H3C + O Na Sodium acetate Carboxylate anions 56 Carboxylate anions O O NaOH or C O C O KOH H Na+ or K+ Hexanoic acid Carboxylate anions: sodium or potasium hexanoate Name of the metal cation (e.g: sodium) + Parent (e.g. hexane) + -ate (suffix) 57 Conversion of carboxylic acids to esters and amides H2SO4 O Formation of ester O H-OR' C R O H Carboxylic acid R alcohol C O H Carboxylic acid R O H-NH2 R O ester O Formation of amide H-OH C ammonia H-OH C R NH2 amide 58 Esters • A modified carboxyl group • Generic formula: RCOOR’ OH and H combined to form H2O O Carboxylic acid C R H O H O R' alcohol O O C R O R' C O R 59 O R' Naming esters • IUPAC system suffix –ate • Two parts 1. Name the acyl group (RCO-) by changing the –ic ending of the parent carboxylic acid to the suffix –ate. 2. Name the R’ group bonded to the oxygen atom as an alkyl group. O Acyl group C R 60 O R' Alkyl group Naming esters O 4 C butanoic acid butanoate O O H2 C H3C C C H2 Methyl butanoate 61 CH3 O R’ = methyl Physical properties of esters O O OH Carboxylic acid 62 O ester Physical properties of esters • Polarity? • Dipolar interaction? • Hydrogen bonding between molecules of same compound? • Hydrogen bonding with water? O • Solubility in water? • Boiling point? O 63 Ester formation from carboxylic acids • Substitution – All acyl (RCO) compounds. O O H-Y C R Z = OH, OR', NR'2 64 R Z Y replaces Z H-Z C Y Y=OH, OR', NR2' Ester formation • Fischer esterification – Treatment of carboxylic acid (RCOOH) with an alcohol (R’OH) in the presence of an acid catalyst forms an ester (RCOOR’). – example O O H-OCH2CH3 C H3C OH Acetic acid 65 H-OH C H3C OCH2CH3 ethyl acetate REFERENCES • Crowe, J., Bradshaw, T. and Monk, P. (2006), Chemistry for the Biosciences: The Essential Concepts, Oxford University Press, Oxford. • Horton, H.R., Moran, L.A., Scrimgeour, K.G., Perry, M.D. and Rawn J.D. (2006). Principles of Biochemistry, 4th Edition. Pearson International Edition. • Smith, J.G. (2010). General, Organic and Biological Chemistry. McGraw-Hill Higher Education. • Denniston, K.J., Toping, J.J. and Caret, R.L. (2008). General, Organic and Biochemistry, 6th edition. McGraw-Hill Higher Education. MY PROFILE Dr Nik Ahmad Nizam Bin Nik Malek, BSc (Ind. Chem.)(UTM), MSc (Chem)(UTM), PhD (Chem)(UTM), A.M.I.C Senior Lecturer, Department of Biotechnology and Medical Engineering, Faculty of Biosciences and Medical Engineering, Universiti Teknologi Malaysia. Email: [email protected], [email protected] Website: http://www.staff.blog.utm.my/niknizam/