* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download File - Serrano High School AP Biology
Photosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
Microbial metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Biochemistry wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
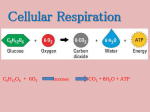
Fermentation We know that early organisms looked like bacteria, but how did the first living cells make the energy they needed? In a primitive environment, with no ozone layer--no barrier against ultraviolet light organic molecules must have flourished. The first cells probably lived as heterotrophs. Heterotrophs need to eat or ingest molecules to make energy. With the help of enzymes, these cells degrade complex organic compounds that are rich in potential energy into simpler waste products that have less energy. This process is a catabolic process. The energy required came in the form of ATP (adenosine tri phosphate). In experiments designed to simulate conditions of 2.6 - 3.9 billion years ago (early Archean), researchers have readily obtained ATP from simple gas mixtures and phosphate. The first cells could have obtained their energy by ingesting ATP. As the bacteria flourished and reproduced, the supply of ATP became depleted. Cells had to have another method for producing ATP for themselves. Since there was no oxygen, the process was anaerobic (without oxygen). The process that was used is called Anaerobic Respiration or Fermentation. Background Information: A) Energy: Energy is the capacity to do work. Energy exists in many forms and life depends on changing energy from one form into another. Kinetic energy is the energy of movement. Heat is a form of kinetic energy; it is the movement of molecules. Potential energy is the energy of location. Electron location is potential energy. B) Thermodynamics is the study of energy transformation. 1) First Law of Thermodynamics: Energy can be changed from one form into another, but cannot be created nor destroyed. Energy can be stored in various forms then changed into other forms. For example, energy in glucose is oxidized to change the energy stored in chemical bonds into mechanical energy. In all energy conversions some of the useful energy is converted to heat and so dissipates. Scientists have developed the notion of potential energy, which is "stored" energy. Molecules contain potential energy in bonds. When the bonds are broken, other bonds form, and some heat is always produced. 2) Second Law of Thermodynamics: In all energy exchanges and conversions, it is proven that if no energy leaves or enters the system under study, the potential energy of the final state will always be less than the potential energy of 1 the initial state. If the reaction releases energy, then the potential energy of the final state is less than the potential energy of the initial state. This type of reaction is called an EXERGONIC REACTION. These reactions occur without any energy being added. (A B + Energy) 3) ENDERGONIC REACTIONS need energy to complete the reaction. The energy added is greater than the difference between the reactants and the products. (Energy + A B) Another factor besides the gain or loss of heat affects the change in potential energy-ENTROPY. Entropy means the disorder of a system. The final state has more entropy and less potential energy than the initial state. The second law states that in other terms all natural processes tend to proceed in such a direction that disorder/randomness increases. C) Free Energy: Spontaneous process is a change that can occur on its own. A non-spontaneous process is a process that needs energy. In any system a spontaneous process increases the stability of the system (stability for proteins is bad—since proteins are highly ordered, they are unstable. Energy is needed to maintain the highly ordered structures. A pile of rubble is more stable than a house. As you move to stability (entropy), you basically increase disorder of a highly ordered system.). But we reviewed that a spontaneous process increases disorder. How can we resolve this difference? Free energy is a portion of a system’s energy that can perform work when the temperature is uniform throughout the system. The quantity of free energy in a system is G. G is made up of 3 components: H = total energy of a system, S = entropy, and T = temperature in Kelvin. If there is an increase in temperature, there is an increase in random motion of molecules, which increases disruption. The equation: G = H - TS (This is the Gibbs Free Energy Equation. This can explain a bunch of concepts e.g. old age, how proteins denature with increased heat…) Think of G as a measure of a system’s instability. Systems that are rich in Energy are unstable. Highly ordered systems, such as proteins, are also unstable. For a process to occur spontaneously it must decrease or S must increase. As G decreases, the greater the likelihood of a spontaneous reaction (remember spontaneous reactions are bad for highly ordered systems. That means the system moves to stability.). Which brings us to the two types of reaction: exergonic and endergonic. Exergonic reactions are reactions in which there is a decrease in G. These reactions occur spontaneously. In endergonic reactions, free energy is absorbed, the energy is stored and the G increases. This reaction needs energy to reaction. The cell uses the exergonic reactions to do the endergonic reactions. ATP releases energy, which is passed to the molecules to react. 2 D) Oxidation/Reduction Reactions: The reactions that occur when an atom gains or loses one or more electrons are called oxidation/reduction reactions. The use of chemical energy in living organisms involves oxidation/reduction reactions. Oxidation is the loss of an electron, i.e. Fe2+ -----> Fe3+ + 1e-. The Fe2+ ion has been oxidized. The ion has lost an e- and a negative charge. Reduction is the gain of an electron. i.e. O + e- ----> O-. When the oxygen receives an electron, it gains a negative charge. Some compounds can accept and donate electrons readily, and these are called electron carriers in organisms. There are a number of molecules that serve as electron carriers. One molecule is NAD+ (Nicotinamide adenine dinucleotide) and is used in anaerobic respiration. NAD+ has a positive charge due to a nitrogen atom. NADP+ (Nicotinamide adenine dinucleotide phosphate) is another used in photosynthesis. These molecules readily give up 2 e- (oxidized) and gain 2 e(reduced). The dehydrogenase enzyme will remove 2 H from a molecule and give 2 electrons and 1 H+ to NAD+. The other H+ is released into the environment. The NAD+ becomes NADH (neutral). NADH has stored energy from the electrons. When the electron moves to a lower energy level, energy is released. It takes energy to remove electrons from an atom. Organic molecules that have a lot of hydrogens are excellent fuels. Hydrogen is an atom, which is used as a source of electrons. E) ATP: Cells need energy to drive reactions. The molecule that supplies the energy is ATP (This reaction is called ATP HYDROLYSIS). The three phosphates on ATP are all negatively charged and bonded together. The like charges cause the phosphates to repel each other, but the covalent bond keeps the molecules together. This situation makes ATP to act as a loaded spring. When the third phosphate is removed by hydrolytic cleavage, part of the spring is released, and 7 kcal of energy is released per mole of ATP. As in Goldilocks, the 7 Kcal of energy is the just the right amount of energy to drive reactions in the cell. (If Glucose were oxidized, then 686 Kcal of energy would be released. This is too much energy!) ATP + H2O ------> ADP + Phosphate + Energy (7 Kcal). When the second phosphate is removed, the same amount of energy is released. ADP + H2O ------> AMP + Phosphate + Energy (7 Kcal). The bonds between the two phosphates are not strong bonds. In fact, these bonds are easily broken releasing 7 Kcal of energy per mole. 7 Kcal of energy is enough to drive endergonic reactions in the cell. All the energy does not come from the moving of electrons to a lower energy level. In fact, the 3 rearrangement of electrons in other orbitals (i.e. ATP --> ADP) results in a structure with less energy. Enzymes catalyzing the hydrolysis of ATP are ATPases. Sometimes the terminal phosphate group is transferred to another molecule. The addition of a phosphate group is called PHOSPHORYLATION. Enzymes that catalyze this reaction are called KINASES. In these phosphorylation reactions, energy is transferred from the phosphate group in ATP to the phosphorylated compound. This newly energized compound will participate in other reactions. ATP originates when anaerobic respiration (fermentation) takes place in the absence of oxygen. What happens is that sugar is broken down into smaller molecules and energy is released? The energy is used to generate ATP from ADP and P. ADP + P ----> ATP Sugar --------------------------> smaller molecules The breakdown of the sugar takes place through a series of chemical reactions. Living organisms have developed numerous and different fermentation pathways; however, most organisms use the following Embden-Meyerhoff pathway, named for the two discoverers. The anaerobic respiration pathway takes glucose (C6H12O6) and breaks it down into two molecules of pyruvate (three-carbon compound). This occurs in the cytoplasm of the cell. The pyruvate can take two pathways in anaerobic respiration (this depends on the species of organism. You cannot choose your pathway.): 1) Pyruvate will be converted to alcohol (ethanol) and carbon dioxide. This is called alcohol fermentation and is the basis of our wine, beer and liquor industry. 2) The pyruvate will be converted to lactic acid. This is called lactic acid fermentation. Lactic acid is what makes your muscles burn during prolonged exercise; this process is also used to make yogurt. The overall reaction for alcohol fermentation looks like this: C6H12O6 ---------------> 2 CH3CH2OH + 2 CO2 + Energy F) Anaerobic Respiration: There are two phases in fermentation: The first 6 steps are the energy investment steps and the last steps are the energy production steps. 1) Glucose enters the cell through facilitated diffusion. 2) Glucose ---------------> Glucose-6-P ATP -----> ADP Initially glucose is phosphorylized by ATP. This step keeps the glucose in the cell, and makes glucose more reactive. Enzyme: Hexokinase 4 Net use of 1 ATP 3) Glucose-6-P --------> Fructose-6-P Fructose is an isomer of glucose. Enzyme: Phosphoglucoisomerase 4) Fructose-6-P---------> Fructose-1, 6-P (Fructose 1,6 Biphospate) ATP--->ADP Another phosphorylization. This is an example of reaction coupling. Fructose-6-P will convert back to glucose 6-P. However, if phosphorylated immediately, the anaerobic pathway will continue. Net use of 2 ATP Enzyme: Phosphofructokinase 5) Fructose-1,6-P-------> 2 Glyceraldehyde-3-P The enzyme Aldolase splits the 6-carbon molecule into 2 three-carbon molecules. Enz: Aldolase Pi 6) 2 Glyceraldehyde-3-P -----> 2 Diphosphoglycerate-1,3-P (DPG) 2 NAD ---> 2 NADH The enzyme removes 2 electrons and 2 H+ from glyceraldehyde (oxidizes the compound). The electron carrier NAD+ accepts two electrons and 1H+ from the enzyme. Glyceraldehyde accepts a phosphate (inorganic source—from the phosphorus cycle); an exergonic reaction (_G=-10.3 kcal/mole). Enzyme: Triose phosphate dehydrogenase 7) 2 Diphosphoglycerate -------> 2 phosphoglycerate-3-P (3 PG) 2 ADP ---> 2 ATP A phosphate from Diphosphoglycerate is taken from the molecule and added to ADP to form ATP. Net production: 0 ATP molecules (two used and two produced per molecule of glucose). Enzyme: Phosphoglycerokinase 8) 2 Phosphoglycerate-3-P -----> 2 Phosphoglycerate-2-P (2 PG) Phosphate is transferred to an adjacent carbon. This makes it easier to remove the phosphate. Enzyme: Phosphoglyceromutase 9) 2 Phosphoglycerate-2-P --------> 2 phosphoenolpyruvate (PEP) remove water Water is removed from phosphoglycerate-2-P to form PEP. Enzyme: Enolase 10) 2 Phosphoenolpyruvate -------> 2 Pyruvate (pyruvic acid) 2 ADP ---> 2 ATP The phosphate from phosphoenolpyruvate is removed and added to ADP to form ATP. Net ATP production: 2 ATP Enzyme: Pyruvate kinase 5 There is a need to oxidize NADH to have it return to NAD+ to accept two electrons and a H+ from Triose Phosphate dehydrogenase. If NADH is not changed back to NAD+, fermentation will stop. There are two different ways to do the conversion. 11A) 2 Pyruvate -------> 2 Acetaldehyde + 2 CO2 A carbon and 2 oxygens are removed from pyruvate to form a two carbon compound called acetaldehyde. 12A) 2 Acetaldehyde --------------> 2 Ethanol 2 NADH -----> 2 NAD+ Acetaldehyde accepts 2 electrons and a H+ from the NADH molecule. This addition causes acetaldehyde to be converted to ethanol. Or NADH--> NAD+ 11B) 2 Pyruvate ------------> 2 Lactic Acid NADH donates two electrons to pyruvate, which is converted to lactic acid. In anaerobic respiration, the organism invests 2 ATPs into the process and receives 4 ATPs back. The net gain is 2 ATPs. Fermentation is an inefficient form of making energy. The end products, which are excretions into the environment, can still be converted into simpler compounds, releasing more energy. 6