* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 1 - UCSF Biochemistry
Survey
Document related concepts
Transcript
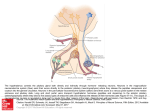
Fly Problem A defining feature of stem cells is that upon division, one daughter can embark on a differentiation pathway while the other daughter necessarily remains a stem cell to maintain the stem lineage. You are interested in investigating the basis of this decision in Drosophila neuroblasts (NBs, neuronal stem cells). The adult drosophila brain contains a structure called the mushroom body, which is generated by many generations of asymmetric divisions by mushroom body neuroblasts (mNBs). At each division one daughter is again a NB while the other daughter is a ganglion mother cell (GMC), which divides once to produce two neurons. The type of neurons generated is determined by NB type and the birth order of the GMC. You have a GAL4 driver line, neuro5GAL4, that is expressed specifically in mNBs. You also have a UAS-GFP (homozygous viable insert) that produces a stable protein that, when driven by neuro5GAL4, marks the four mNBs green; however, because this GFP is stable it will also mark daughter cells originating from the mNBs even though neuro5GAL4 is no longer expressed in these cells. You also have an unstable UASCherry (also homozygous viable) that marks only the four mNBs red (but will not mark daughter cells). Looking at newly hatched larvae, you see four red cells in the brain, two on the left and two on the right, mNB-L and mNB-R. These four mNBs are also seen in the GFP channel and each is associated with a chain of green only cells. The two anterior mNBs, mNB-La and nMB-Ra, have ten cells in their chains and the posterior pair, mNB-Lp and mNB-Rp, have six cells. Illustration of system: Note that the description and tools are slightly contrived, so you won't find a precise mimic of this situation in the literature. This image is provided to help you visualize what is going on. NB division into GMC and NB is physically (size wise) asymmetric (left shows a NB at late mitosis/early cytokinesis stained for proteins that are asymmetrically distributed to the larger daughter NB and smaller GMC). Visualization of the developing mushroom body according to the description would look something like the image below. You want to define genes that control the asymmetry of the division of the mNBs. The Vienna Drosophila RNAi center (VDRC) houses a resource of 22,000 fly lines, each with a UAS driven "gene" that makes a hairpin RNA that folds back on itself to produce dsRNA that get processed to RNAi. The different lines target different genes. In all 88% of the genes in the fly are targeted. You have been invited to Vienna to carry out a screen of this resource. a) What would you do to screen for RNAi lines that target a gene involved in asymmetric division of the mNBs? Most of the inserts in the RNAi library are homozygous viable (without driver). Describe the cross that you would do with these viable lines that are provided as homozygotes to test for an involvement of the cognate gene in the division program of mNBs and describe what you would look for in screening the cross. Note that in answering this question, you are invited to invent different phenotypes that you might see, and your answer might affect how you respond to subsequent parts of this question. b) While your real interest is in genes involved in directing the fate decisions of the mNB daughter cells, you might get many genes that are required for survival of the mNBs, or are otherwise are required nonspecifically for mNBs to divide. Tell me at least two (and not more than four) things that you might do to validate your hits as being involved specifically in the fate decision of mNB daughter cells rather than being involved in more general processes such as cell division or cell survival. These two to four things might be features in the screen itself, or tests run after the identification of candidates. c) Because you are worried about the efficiency and specificity of the RNAi screen, you left your technician at home to do a screen for EMS mutations disrupting the mNB divisions. The plan is to do an F1 screen using MARCM to homozygose newly induced mutations specifically in the mNBs. In addition to the above tools, you have a UAS-Flip, stocks with chromosomes with FRT at the base of each chromosome arm and inserts of GAL80 on each chromosome arm. Describe for one chromosome arm (say 2R) how your technician would conduct this screen. For this problem, you can assume that you can combine any of your tools (inserts) on any chromosome you want without describing how you make the various recombinant chromosomes. Start with the flies that would be mutagenized, and described the crosses and how you would screen for a mutant. For this screen, assume that flies are alive and fertile even with a totally messed up mushroom body and that you can see your GFP and Cherry markers in living larvae. d) You find 18 RNAi lines that give really interesting phenotypes, and your technician finds 11 EMS induced mutations that give similarly interesting phenotypes in the mNB lineages when examined as clones. Of the 11 EMS mutations, 9 are lethal and these fall in 5 complementation groups. When homozygous, the two non-lethal mutations give viable flies with a messed up mushroom body. Transheterozygotes of these viable mutations have the same messed up mushroom bodies, and tests in trans with the lethal alleles reveal that these mutations define a sixth complementation group (i.e. rather than weak alleles of one of the other complementation groups). You want to know what genes are mutated in these EMS mutants and you expect that the effected genes may be among those identified in your RNAi screen. How would you find out which if any corresponded? Mouse Problem In previous work your lab has identified a gene, toohot, in humans, homozygous deletions of which lead to anosmia (inability to sense odors). In addition, these patients cannot tolerate warm weather such that they get feverish as soon as the fog lifts at Parnassus heights. There is a toohot ortholog encoded entirely by a single exon in mice, and your job is to figure out how absence of Toohot might lead to anosmia and the intolerance to heat. In situ hybridization (ISH) in adult mice reveals that toohot is expressed in sensory neurons of the main olfactory epithelium (MOE) and in a cluster of neurons in the hypothalamus, and nowhere else in the brain. A time course ISH study shows early expression of toohot in the embryo in the olfactory placode (thought to be a precursor to the MOE and some undefined neurons in the hypothalamus). Please be specific about the genetic strategies (transgene vs knock-in) and do provide essential details of the constructs. Assume you have access to a ubiquitous promoter/locus. You hypothesize that the Toohot-expressing neurons in the hypothalamus and the MOE arise from the Toohot-expressing cells in the olfactory placode. 1a) Design a genetic strategy to test this idea. 1b) Please design the appropriate breeding strategy to generate experimental and control mice. What is(are) the genotype of the critical control mouse for this experiment? Why? How would you test that this control works as expected? You knock-out toohot in the mouse and recapitulate the human phenotype - anosmia and extreme intolerance to heat. You’re worried however that the heat intolerance might result from transient/weak expression of Toohot in non-neural tissue such as muscle, which is also important for body temperature homeostasis. You therefore want to test whether Toohot functions in the hypothalamus to regulate heat sensitivity. 2. Design a genetic strategy to test whether toohot functions in the hypothalamus to control heat intolerance. Safe to assume that you have access to a brain or hypothalamus specific promoter/locus. Your expts reveal that toohot in the brain is essential for mediating heat sensitivity. The mice are otherwise perfectly normal in every other biological process. You now wish to test whether Toohot-expressing neurons in the hypothalamus also regulate other physiological processes (besides temperature control). 3a) Why is this still a relevant experiment given that your preceding study showed Toohot to be essential only for temperature control? 3b) Design two distinct genetic strategies to specifically ablate the Toohot+ neurons in the adult hypothalamus.