* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 6.4 The Building Blocks of Life
Gaseous signaling molecules wikipedia , lookup
Protein adsorption wikipedia , lookup
Genetic code wikipedia , lookup
Expanded genetic code wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Proteolysis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Metalloprotein wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
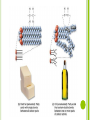
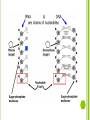
CHAPTER 6.4 The Building Blocks of Life ORGANIC CHEMISTRY Life on Earth is carbonbased Carbon is a component of almost all biological molecules Lead to the diversity of life on Earth Organic chemistry: Student of organic compounds (compounds containing carbon) ORGANIC CHEMISTRY Carbon has four electrons in its outermost energy level so carbon can form four covalent bonds with other atoms Compounds can be in the shape of straight chains, branched chains, and rings MACROMOLECULES Large molecules that are formed by joining smaller organic molecules together Large molecules are called polymers Molecules made from repeating units of identical or nearly identical compounds called monomers that are linked together by covalent bonds Poly=many Mono=one Four types: Carbohydrates Lipids Proteins Nucleic Acids CARBOHYDRATES Compounds composed to carbon, hydrogen, and oxygen in a ratio of one oxygen and two hydrogen atoms for each carbon atom General formula: (CH2O)n n=the number of CH2O unites in a chain 3-7 are called simple sugars or monosaccharides Two monosaccharides join together to form a disaccharide Ex: Glucose Ex: Sucrose Larger molecules are called polysaccharides Ex: Glycogen CARBOHYDRATES In plants, a carbohydrate called cellulose provides structural support in cell walls Chitin, a polysaccharide, is the main component in the hard outer shell of shrimp, lobsters, and insects LIPIDS Molecules made mostly of carbon and hydrogen that make up fats, oils, and waxes Composed of fatty acids, glycerol, and other components Main function is to store energy Ex: Triglyceride Fat if solid at room temperature and oil if liquid at room temperature Stored in fat cells in the body SATURATED AND UNSATURATED FATS Basic structure of a lipid: Fatty acid tail Chain of carbon atoms bonded to hydrogen and other carbon atoms by single or double bonds Single bonds: Saturated fats Double bonds: Unsaturated fats Saturated fats: No more hydrogens can bond to the tail Unsaturated fats: t least one more hydrogen can be accommodated in the tail Polyunsaturated fats: Fats with more than one double bond in the tail PHOSPHOLIPIDS Responsible for the structure and function of the cell membrane Lipids are hydrophobic They do not dissolved in water Allows lipids to serve as barriers in biological membranes STEROIDS Substances such as cholesterol and hormones Cholesterol provides the starting point for other necessary lipids such as vitamin C or hormones such as estrogen and testosterone PROTEINS Compound made of small carbon compounds called amino acids Amino acids are small compounds that are made of carbon, nitrogen, oxygen, hydrogen, and sometimes sulfur AMINO ACID STRUCTURE Central carbon atom Can form four bonds One bonds with hydrogen Other three bond with an amino group (-NH2), carboxyl group (-COOH), and a variable group (-R) Variable group makes each amino acid different 20 of them Proteins are made of different combinations of all 20 different amino acids Several covalent bonds called peptide bonds join amino acids together to form proteins THREE-DIMENSIONAL PROTEIN STRUCTURE Proteins can have up to four levels of structures due to the variable groups Number of amino acids in a chain and the order in which they are joined, define the primary structure Secondary structure: Helix and pleat Tertiary structure: Globular Can contain many Ex: Hemoglobin Quaternary structure: Long fibers PROTEIN FUNCTION Make up 15% of total body mass Involved in nearly every function of your body Structural support Transport substances inside the cell and between cells Communicate signals within the cell and between cells Speed up chemical reactions Control cell growth Ex: Skin and hair made of proteins NUCLEIC ACIDS Complex macromolecules that store and transmit genetic information Made of smaller repeating units called nucleotides Composed of carbon, nitrogen, oxygen, phosphorous, and hydrogen atoms Six nucleotides Three units: nitrogenous base, phosphate, ribose sugar Two nucleic acids in living organisms: DNA and RNA Nucleotide with three phosphate groups: Adenosine Triphosphate (ATP) Chemical storehouse of chemical energy DNA AND RNA Deoxyribonucleic acid (DNA) Ribonucleic acid (RNA) Sugar of one nucleotide bonds to the phosphate of another nucleotide The nitrogenous base that sticks out from the chain is available for hydrogen bonding with other bases